NMR1. Introduction.
Magnetic resonance imaging is a medical diagnostic technique that uses radio waves to learn about the environment of hydrogen atoms in your body. As it happens, the hydrogen atoms do not respond to the radio waves unless there is a strong magnetic field present. Looking at the hydrogen atoms in your body gives the technician and the doctor information on any of the soft tissues of the body, since they are largely composed of water and contain lots of hydrogen atoms.
Nuclear magnetic resonance (NMR) is a technique that is very closely related to magnetic resonance imaging. The term "nuclear" refers to the fact that the radio waves interact with the nucleus of the hydrogen atom, or other atoms that you might be interested in studying. NMR spectroscopy is probably the most important tool available for determining the structure of organic compounds because it tells you what atoms are present and how they are connected to each other.
The key information that we can receive from NMR is the number of inequivalent atoms of a particular kind that are present in a compound. For example, If we analyze a sample of a carbon-containing compound, we can identify the number of carbon atoms that are found in distinct positions within the molecule. The following chart illustrates how different carbon atoms within a structure occupy distinct positions within a structure. These positions are different from each other; hence the term, inequivalent.
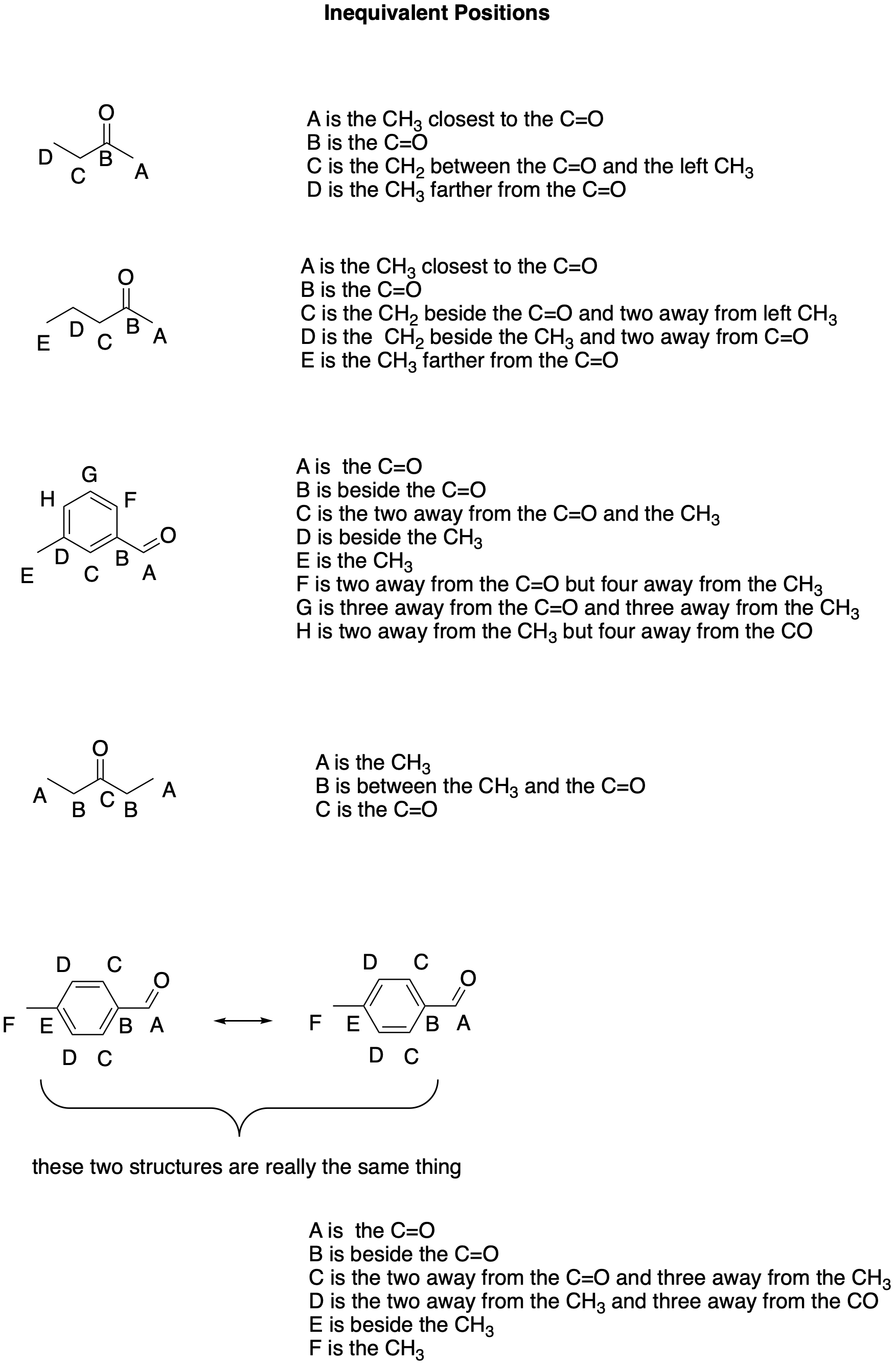
Figure NMR1.1. The concept of inequivalency illustrated with carbon atoms.
In some cases, each carbon in the structure is in a position like no other carbon in the structure. NMR would tell us that there are four different carbons in the first compound, five in the second, and eight in the third. However, in the last two examples, there is symmetry within the molecule. In those cases, there are examples in which two carbon atoms occupy positions indistinguishable from each other. These equivalent carbons would not be detected as different in NMR. NMR would find only three carbons in the fourth structure and six carbons in the fifth.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: