Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
NMR11. More Complicated Coupling
The n + 1 rule (number of lines in a multiplet = number of neighbouring H + 1) will work for the majority of problems you may encounter. Occasionally, you may see more complicated coupling. The spectrum of methyl acrylate is a good example. There are a couple of points to note in this spectrum, beginning with the number of peaks.

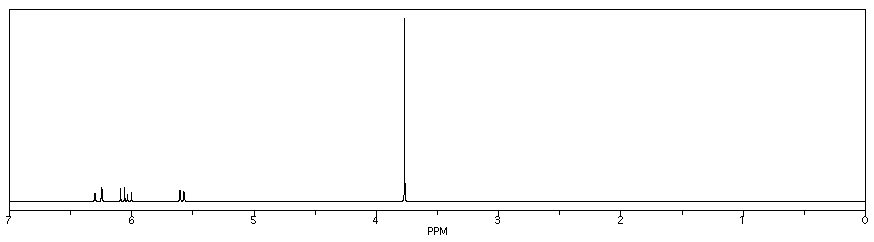
Figure NMR11.1. Simulated 1H NMR spectrum of methyl acrylate.
In addition, there is a problem with coupling in the vinyl region.
This pattern is called a "doublet of doublets". The two symmetry-inequivalent neighbors on the other end of the double bond each act as if the other one isn't there. They couple to the proton next to the carbonyl independently, each one splitting the peak for this proton into a separate doublet.
There are a few cases in which this independent coupling will occur rather than the (n+1) type coupling we saw first. Generally, independent coupling occurs when protons are not freely rotating. That can happen if one of the protons is attached to a double-bonded carbon, because we can't rotate around a double bond. It may also happen with protons that are directly attached to the carbons of a ring.
To see why this happens, you need to know more about coupling.
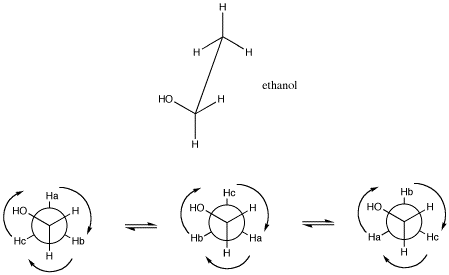
Figure NMR11.2. A sawhorse projection (top) and Newman projection of ethanol (bottom), showing the angular relationship between hydrogens on neighbouring carbons. The angle between two groups on neighbouring carbons is called the dihedral angle. Because of free rotation about the C-C bond, all three hydrogens on the methyl have an equivalent spatial relationship to the hydrogens on the CH2 group.
Sometimes coupling information is depicted as an arrow. This arrow stands for the coupling constant between two protons. The coupling constant is related to the spin of a hydrogen atom. The spin (related to magnetic moment) can be aligned with the external magnetic field (we will show it pointing up) or else against it; no other possibilities are allowed.
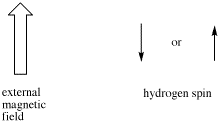
Figure NMR11.3. Proton spins in an external magnetic field.
If there are two neighbouring hydrogens, both spins could be aligned with the external field, both could be aligned against it, or one could be aligned each way. That means there are three different magnetic combinations that will each have a different effect on the observed proton: increased magnetic field, decreased magnetic field, and no net effect (canceling out).
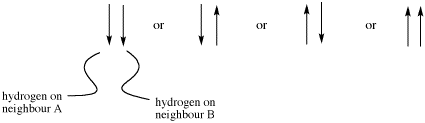
Figure NMR11.4. Combinations of spins of two different protons.
These three combinations result in the observed proton absorbing at three different frequencies, because the frequency it absorbs is sensitive to the magnetic field it experiences. Note that there are two ways to arrive at the middle possibility, with one neighbour spin up and the other spin down. Statistically this possibility is twice as likely as either both spins up or both spins down. It is thus twice as likely that the observed proton experiences that effect, and so the middle line in a triplet is twice as high as the other two lines.
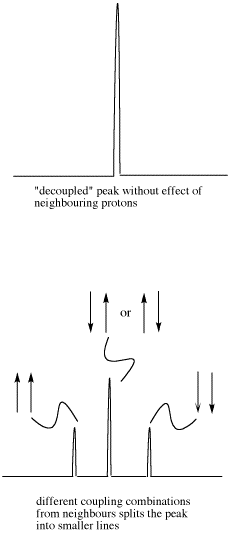
Figure NMR11.5 Effects of neighbouring protons on an observed peak. This case assumes all the neighbouring protons have an equivalent effect (they have the same coupling constant with the observed proton).
However, the size of that arrow, the coupling constant, is only the same for two neighbouring hydrogens if they have the same spatial relationship with the observed hydrogen. That isn't always true.
As a result, the two coupling constants are different. We can depict that situation using arrows of different lengths for the two neighbouring proton spins. Each spin can be either up or down, but now two opposing spins do not cancel out. The result is four spin combinations of equal probability, not just three.
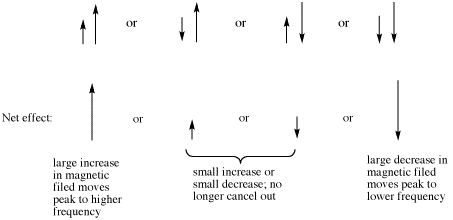
Figure NMR11.6. Combinations of two neighbouring spins with different coupling constants.
The doublet of doublets is four lines of about equal heights. The distance between the two pairs of lines on each edge
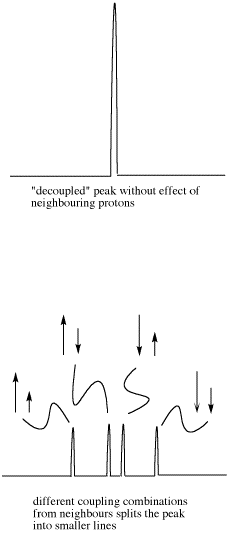
Figure NMR11.7. Effects of neighbouring protons on an observed peak. This case assumes neighbouring protons have inequivalent effects (they have differing coupling constants with the observed proton).
The dihedral angle is limited in only a few specific cases:
Problem NMR11.1.
Sketch spectra for the following compounds.
a) allyl alcohol or 2-propene-1-ol, HOCH2CH=CH2
b) styrene or vinylbenzene, C6H5CH=CH2
c) trans-1-chloropropene, CH3CH=CHCl
Problem NMR11.2.
A compound was shown via high-�‐resolution mass spectrometry to have the probable formula C6H10O2.
a) What is the degree of unsaturation in this compound?
b) IR spectroscopy gave the following data: 3105 (w), 2950 (m), 1517 (m), 1235 (s), 1056 (s), 715 (m) cm-1. Provide a data table with possible assignments for these peaks.
c) 1H NMR spectroscopy provided the following spectrum. Provide a data table with partial structures for each peak.
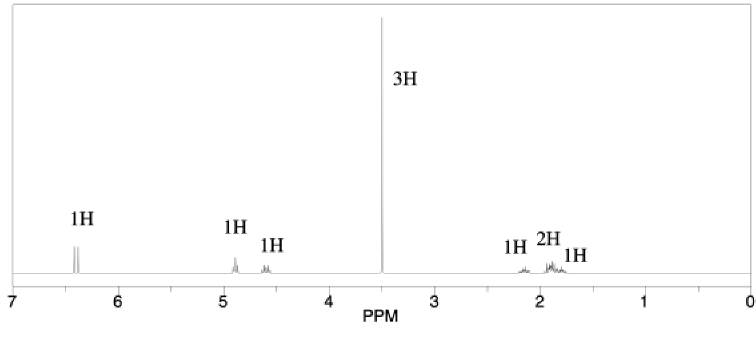
d) Suggest a likely structure for this compound.
Problem NMR11.3.
A compound was shown via high-�‐resolution mass spectrometry to have the probable formula C5H8O2.
a) What is the degree of unsaturation in this compound?
b) IR spectroscopy gave the following data: 2950 (m), 2825 (m), 2716 (m), 1724 (s), 1505 (m), 1056 (s) cm-1. Provide a data table with possible assignments for these peaks.
c) 1H NMR spectroscopy provided the following spectrum. Provide a data table with partial structures for each peak.
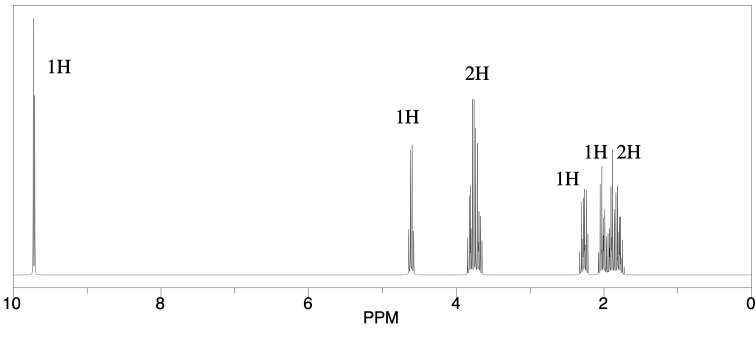
d) Suggest a likely structure for this compound.
Problem NMR11.4.
A sample of a natural product was isolated from plant material and subjected to analysis. The compound was shown via high‐resolution mass spectrometry to have the probable formula C10H12O.
a) What is the degree of unsaturation in this compound?
b) IR spectroscopy gave the following data: 3097 (m), 2975 (m), 1600 (m), 1495 (m), 1235 (s), 1056 (s), 747 (m), 705 (m) cm-1. Provide a data table with possible assignments for these peaks.
c) 1H NMR spectroscopy provided the following spectrum. Provide a data table with partial structures for each peak.
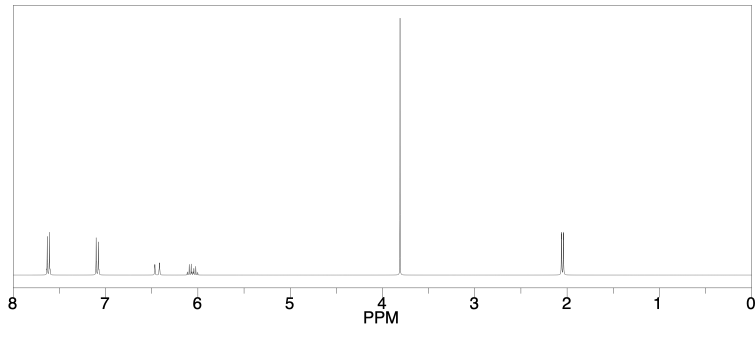
d) Suggest a likely structure for this compound.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: