NMR4. Factors in Chemical Shift: Carbon Geometry
As in IR spectroscopy, the frequency at which
different carbons absorb in the NMR spectrum is pretty predictable. There are
two main factors that control the shift. These two factors are :
- the geometry around the carbon atom.
- the electronegativity of the other atoms attached to the carbon.
First we'll look at the influence of geometry.
In all of the hydrocarbon spectra seen above, the only
peaks observed are at the right-hand end of the spectrum. The 13C
spectrum ranges from about 0 ppm to about 200 ppm, and all of the peaks we
observed were well below 100 ppm. One of the things that those compounds
had in common (cyclohexane, butane, pentane) was that they contain only
tetrahedral (or sp3) carbons. And, in fact, that kind of carbon
atom generally shows up below 100 ppm (typically below 80 ppm) in the 13C
spectrum.
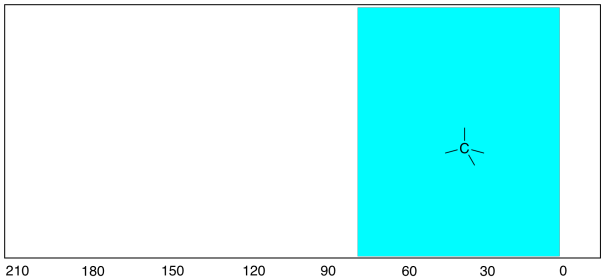
Figure NMR4.1. The tetrahedral carbon or sp3
region of the 13C NMR spectrum.
That's an important trend to know, because tetrahedral
carbons are quite common in nature. Another shape of carbon atom, trigonal
planar, is also pretty common. In contrast to the tetrahedral carbons,
these carbons show up in the left-hand half of the spectrum, between 100 and 200
ppm.
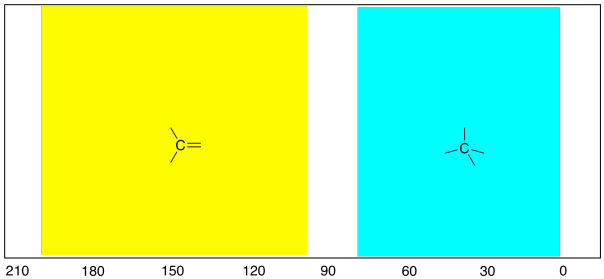
Figure NMR4.2. Comparison of the trigonal planar or
sp2 region with the tetrahedral or sp3
region of the 13C NMR spectrum.
As a general rule:
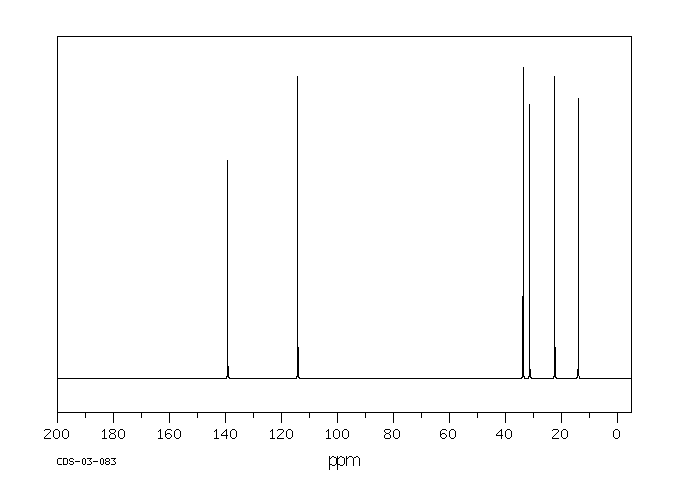
The alkene, 1-hexene, shows four peaks below 20 ppm
and two near 100 ppm. The two carbons that absorb near 100 ppm are the two
trigonal carbons that form the alkene functional group; the others are
tetrahedral carbons.
Remember, a general rule means that there will be
exceptions. You might see sp2 carbons at 210 ppm or 95 ppm, for
example. You are not likely to see them at 30 ppm, however.
There are some more subtle factors that influence the
shift of a carbon atom in the spectrum, having to do with what the neighbouring
groups are in the compound. For example, a tetrahedral carbon shifts a
little bit downfield if it is next to a trigonal planar carbon. The reason
has to do with the way the magnetic field that is used in this experiment
recruits the electrons of double bonds, essentially magnetizing them, as well.
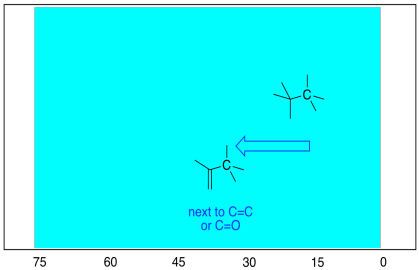
Figure NMR4.4 The downfield shift of a carbon
next to a double bond in the 13C NMR spectrum.
The same thing happens if trigonal planar carbons are near other trigonal planar
carbons. Just as in the tetrahedral case, these sp2 carbons can
be pulled to the left under the influence of neighbouring double bonds. As
a result, carbons in benzene rings show up slightly to the left of carbons in
alkenes.
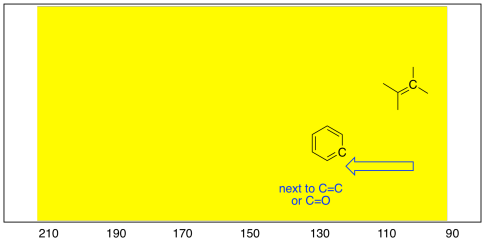
Figure NMR4.5.The difference between arene (aromatic or benzene)
carbons and alkene carbons in the 13C NMR spectrum.
- sp2 carbons in alkenes show up near the upfield end of the sp2
half of the spectrum (usually around 100-120 ppm).
- sp2 carbons in aromatic systems like benzene absorb a little
farther downfield than alkenes (around 120-160 ppm).
Another compound that contains both sp2 and
sp3 carbons is toluene or methylbenzene. Its 13C NMR
spectrum shows one peak near 30 ppm and four between 120 and 130 ppm.
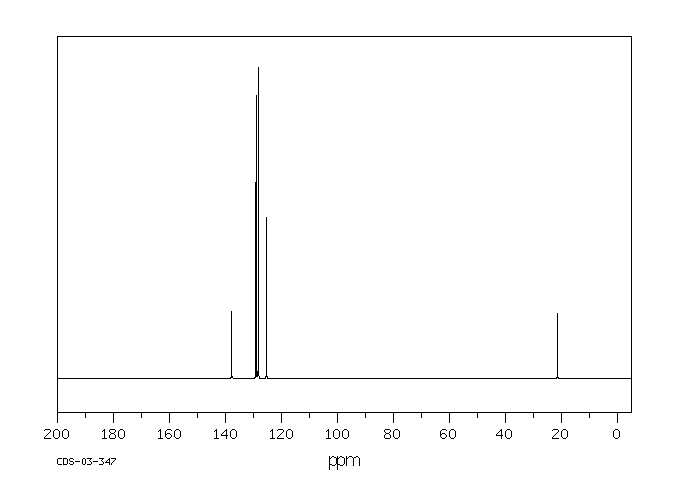
Figure NMR4.6.The 13C NMR spectrum of toluene.1
Problem NMR4.1.
Draw an approximate 13C NMR spectrum
for each of the following compounds. You will need to take into account symmetry
and carbon geometry.
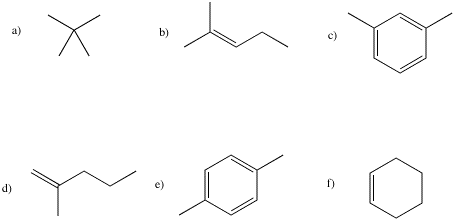
Problem NMR4.2.
Determine whether the following
peaks represent sp2 (trigonal planar) or sp3 (tetrahedral)
carbon atoms.
a) 27 ppm
b) 198 ppm c) 112 ppm
d) 15 ppm e) 79 ppm
f) 164 ppm
In addition, there are also linear carbons in organic
chemistry, although they are much less common as tetrahedral and trigonal
carbons. Linear or sp carbons absorb at about 60 to 100 ppm, sort of in the
middle of the spectrum. Mostly they are seen around 60-80 ppm, so they
overlap considerably with sp3 carbons.
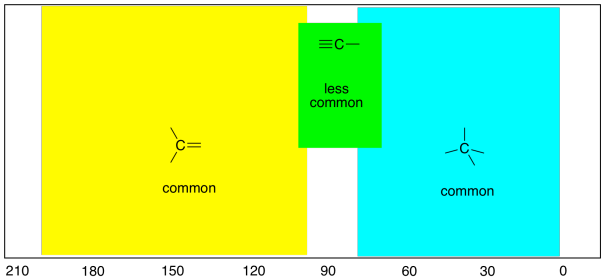
Figure NMR4.6.The sp or linear carbon region in the 13C NMR spectrum.
- sp or linear carbons absorb around 60-100 ppm.
- clearly, some sp3 carbons absorb at frequencies similar to sp
carbons. Be careful.
One example
of a compound containing sp carbons is 1-hexyne. Its spectrum is shown below.
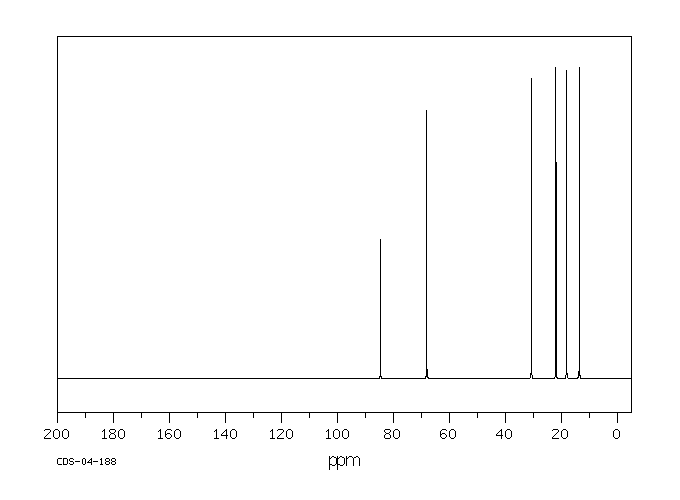
Figure NMR4.7.13C NMR spectrum of
1-hexyne.1
1. Source: SDBSWeb :
http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial
Science and Technology of Japan, 15 August 2008)
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (with contributions from other authors as noted). It is freely
available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Navigation:
Back to NMR
Back to Structure Determination
Back to Structure & Reactivity