Structure & Reactivity
Structure Determination with Spectroscopy
UV4. UV-Visible Spectroscopy: Solutions.
Problem UV1.1.
You observe a coloured object. Estimate the wavelength of light that was absorbed by the object.
a) 700 nm (just an estimate, but maybe somewhere between 620 and 750 nm)
b) 425 nm c) 540 nm d) 580 nm
Problem UV1.2.
a) 590 nm b) 400 nm c) 500 nm d) 300 nm
Problem UV1.3.
a) 590 nm (strong); that's all b) 400 nm (strong); 600 nm (weak)
c) 500 nm (strong); 350 nm (weak) d) 300 nm (strong); 450 nm (weak)
Problem UV1.4.
Remember, here we are observing the photons directly, rather than the onew complementary to the absorbed photons.
copper > barium > sodium > calcium > lithium
Problem UV2.1.
a) 270 nm (strong, MLCT); 500 nm (weak, d-d)
b) < 200 nm* (very strong, MLCT); 275 nm (strong, MLCT); 400 nm (weak, d-d) *can't see exactly where because the peak is so tall that we can't see the top
c) < 200 nm* (very strong, MLCT); 275 nm (strong, MLCT); 660 nm (weak, d-d)
d) < 250 nm (strong, MLCT); 300 nm (strong, MLCT); 620 nm (weak, d-d)
Problem UV2.2.
a) absorbs blue-green; we see orange-red
b) absorbs violet; we see yellow
c) absorbs red; we see green
d) absorbs orange; we see blue
Problem UV2.3.
a) A = ε c l = 14 L g-1 cm-1 x 0.025 g L-1 x 1 cm = 0.35
a) A = ε c l = 14 L g-1 cm-1 x 0.011 g L-1 x 1 cm = 0.15
Problem UV2.4.
a) A = ε c l or c = A / ( ε l ) = 0.75 / (14 L g-1 cm-1 x 1 cm) = 0.054 g L-1
b) A = ε c l or c = A / ( ε l ) = 0.21 / (14 L g-1 cm-1 x 1 cm) = 0.015 g L-1
Problem UV2.5.
Based on our colour wheel it absorbs both yellow and green; we see a mix of violet and red. (KMnO4 is really a deep purple.)
Problem UV2.6.
KMnO4 has MW = 158 g mol-1
14 L g-1 cm-1 x 158 g mol-1 = 2212 L mol-1 cm-1
Problem UV2.7.
a) A = ε c l = 2 200 L mol-1 cm-1 x 0.00015 mol L-1 x 1 cm = 0.33
a) A = ε c l = 2 200 L mol-1 cm-1 x 0.00009 mol L-1 x 1 cm = 0.20
Problem UV2.8.
a) A = ε c l or c = A / ( ε l ) = 0.47 / (2 200 L mol-1 cm-1 x 1 cm) = 2.1 x 10-4 mol L-1
b) A = ε c l or c = A / ( ε l ) = 0.89 / (2 200 L mol-1 cm-1 x 1 cm) = 4.1 x 10-4 mol L-1
Problem UV3.1.
a) blue b) orange
Problem UV3.2.
Add another 30 or 40 nm and you get to 270 or 280 nm.
Problem UV3.3.
a) about 250 nm b) about 280 nm
Problem UV3.4.
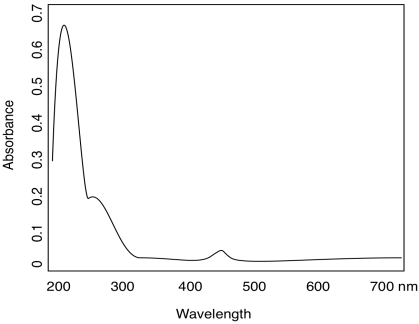
a)

b) yellow
Problem UV3.5.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: