Structure & Reactivity
Introductory Mass Spectrometry
MS2. The Mass Spectrometry Experiment
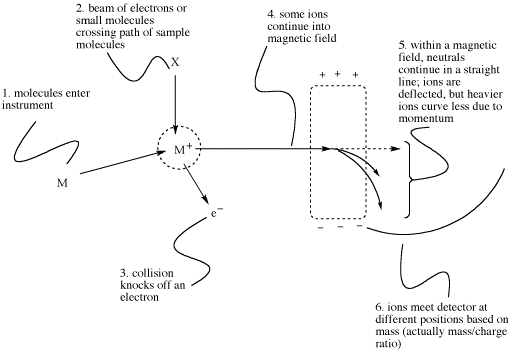
How can we "weigh" something as small as a molecule? In mass spectrometry, the balance between two different physical phenomena can be used to measure mass.
When placed in a magnetic field, a charged particle (such as an electron or proton) will be attracted toward one end of the field. When coupled with the phenomenon of inertia, this magnetic attraction can be used to differentiate between particles with different mass.
Suppose two particles moving parallel to each other enter into a magnetic field at the same time. One of these particles is heavier than the other. Both are deflected a little bit from their original path, but one of the particles -- the heavier one -- does not turn as easily and so it is not deflected as much. If we can measure how far each particle is deflected, we may be able to get an idea of the mass in each case.

Figure MS2. A very approximate schematic of a typical mass spectrometry experiment.
Mass spectrometry only works with ions, not with neutral molecules. That means a neutral molecules must become charged in order to do this experiment. It is common to generate a cation from the molecule by removing one electron. The electron is knocked off the molecule in a collision. The collision can be caused in two different ways:
The data from a mass spectrum shows the abundance of ions of different masses in the spectrometer. Because molecules tend to fall apart under these conditions, many different masses are observed for one molecule. Most of these masses correspond to smaller pieces of the molecule after it has fallen apart. However, because we are always working with very large numbers of molecules when we look at a sample, there is a statistical chance that at least some of the molecules remained intact during the experiment. Also, because we are looking at a population of molecules, we get a graph that tells us the likely fate of the molecule under these conditions. Maybe the molecule has a high probability of falling apart in a certain way to produce a fragment of a certain mass. In that case, we see a high abundance of ions in the spectrometer with that mass. The results look something like the simulated data below.
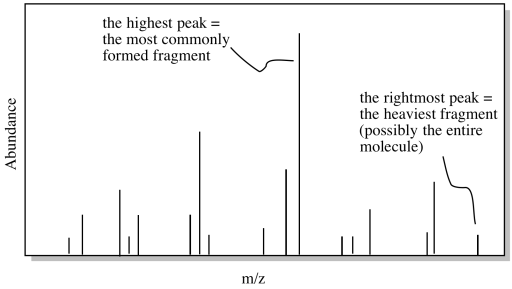
Of course, if the molecule does manage to hold together, then it will be bigger than any of the fragments that would result if it falls apart. The intact molecule will show up at the highest molecular weight, to the right in the graph. All the peaks to the left of it would represent different ways in which the molecule falls apart.
The reason the x-axis on a mass spectrum is labeled m/z (mass-to-charge ratio) is to acknowledge that there are really two factors contributing to the experiment. The "mass" or molecular weight that is measured really is the ratio of the molecular weight of the ion to its charge. In small molecules, the instrument usually measures ions with a charge of +1. In that case, the x axis really shows the mass of the molecule or of a fragment.
However, large molecules such as proteins have many, many sites where ions might form. Most obviously, they have lots of amino groups that might pick up extra protons. In that case, the charge is not just +1. What is often observed is a cluster of peaks representing different charges but related by the molecular weight of a protein.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: