Structure & Reactivity
Introductory Mass Spectrometry
MS6. Isotopomers
If you look closely at the mass spectrum of an organic compound, 2-butanone, you see a line at m/z 72, which corresponds to 4 carbons, an oxygen and 8 hydrogens.

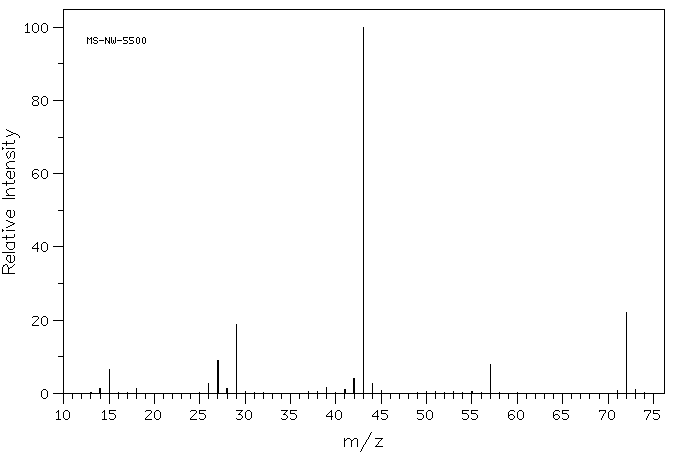
Figure MS3. Mass spectrum of 2-butanone.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 22 August 2008)
In addition, there are a number of other lines at lower values of m/z; these correspond to the masses of smaller pieces of those 2-butanone molecules that fall apart during the experiment. We won't look too closely at how those arise until we get to radical reactions later in the course. However, we will look at some factors that make cations stable later in this chapter.
If you look closely at the mass spectrum of 2-butanone, you'll also see another little peak at m/z 73. This is referred to as the M+1 peak (one greater than the molecular ion), and it arises because of 13C. This compound is referred to as an isotopomer; that means the same compound with a different isotope.
The chance that a molecule in a sample contains a 13C atom is related to the number of carbons present. If there is just one carbon atom in the molecule, it has a 1% chance of being a 13C. That means the M+1 peak would be only 1/100th as tall as M+, the peak for the molecular ion.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: