Structure & Reactivity
Introductory Mass Spectrometry
MS7. Other Important Isotopes: Br and Cl
There are other cases in which knowledge of isotopes can be crucial. For example, bromoethane should display a peak for the molecular ion at m/z = 109. However, the farthest large peak to the right in its mass spectrum is at 110. There is another large peak at 108.

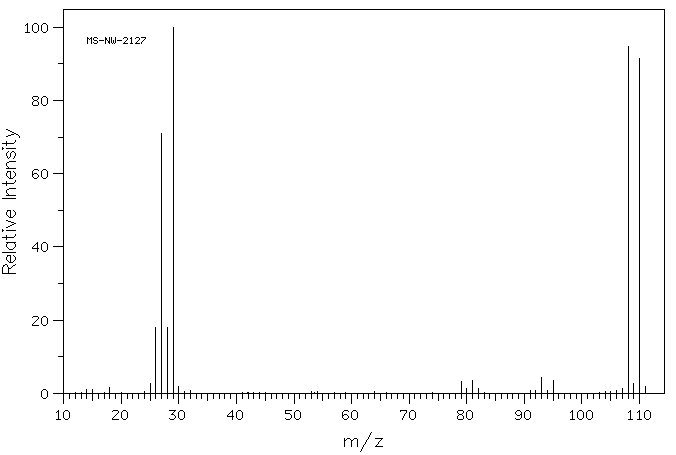
Figure MS4. Mass spectrum of bromoethane.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 22 August 2008)
Similarly, chlorobenzene should display M+ at m/z =112, provided you take into account that most chlorine atoms have an atomic weight of 35 amu. The periodic table lists an atomic weight for chlorine of 35.453 amu, though. That's because about 25% of chlorine atoms are actually 37Cl. The mass spectrum of chlorobenzene actually shows an additional molecular ion at 114 amu.
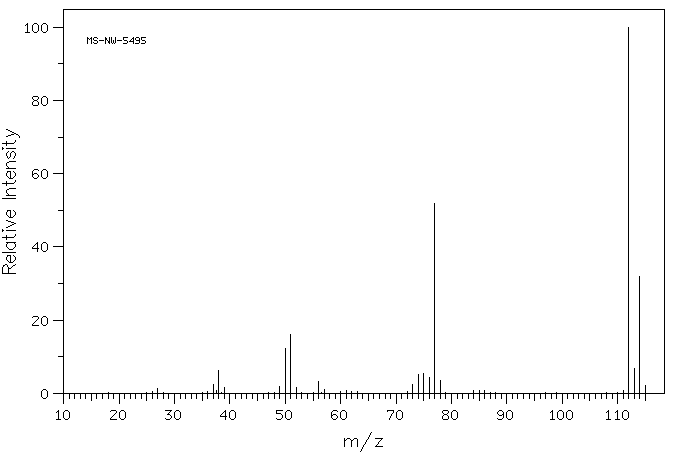
Figure MS5. Mass spectrum of chlorobenzene.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 22 August 2008)
Note also that halogens are easily lost during mass spectrometry. If you subtract the mass of the halogen from the molecular ion mass, you will often find a peak that corresponds to the remainder of the structure.
Problem MS7.1. Draw one possible structure for the compound in each of the following mass spectra.
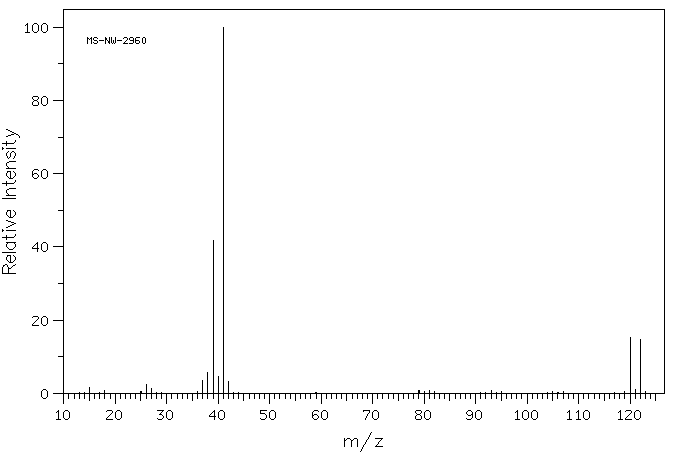
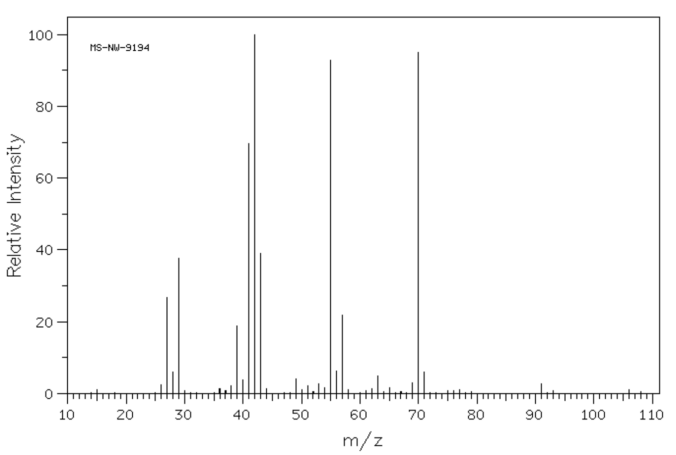
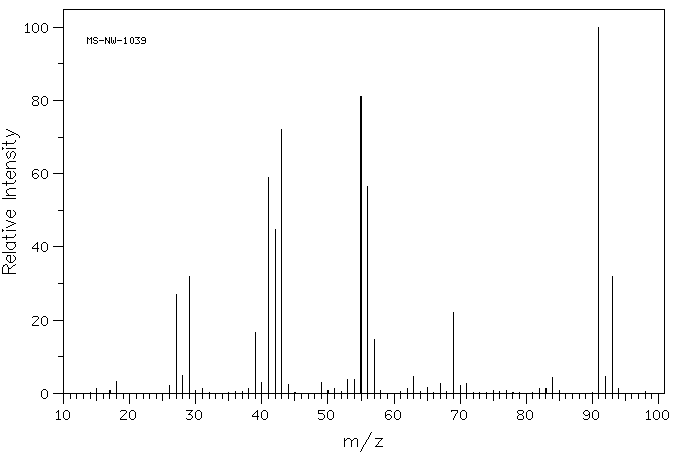
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 25 August 2008)
Problem MS7.2. In the following mass spectrum, more than one halogen atom is present.
a) What is a possible structure of the compound?
b) Show why this pattern of molecular ions is observed.
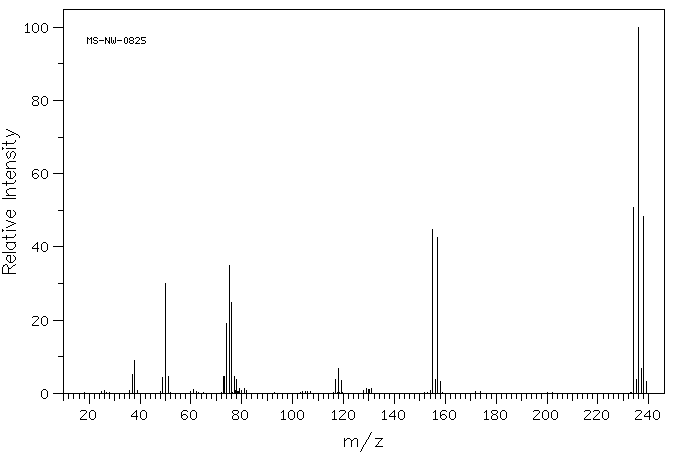
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 25 August 2008)
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: