Structure & Reactivity
Introductory Mass Spectrometry
MS4. Molecular Ions
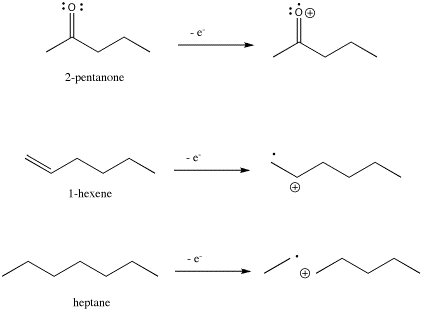
The ion that results from the loss of an electron is called the molecular ion. The molecular ion is an example of a radical cation. It is a cation because of its plus charge, and a radical because not all of its electrons are found in pairs.
Because an electron is so light compared to the mass of all the protons and neutrons in the molecule, the mass of the molecular ion is essentially the same as that of the original molecule.
The molecular ion, of course, has the same structure as the original molecule, minus one electron. But where does the lost electron come from? Usually the following trend is seen:
Thus, in 2-pentanone the electron would most easily be lost from a lone pair; in hexene it would be lost most easily from the pi bond, and in heptane it would be lost most easily from a sigma bond.

Figure MS4. Ionization in three different organic compounds.
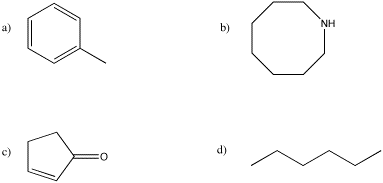
Problem MS4.1.
Draw an equation for the formation of a molecular ion from each of the following compounds.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: