
Structure & Reactivity
Introductory Mass Spectrometry
MS11. Fragmentation Pathways
When molecules go through a mass spectrometer, they break into pieces in a variety of different ways. There are many ways in which the molecules might break apart, but a few of these pathways are especially useful to know.
Alpha Fragmentation
An alpha fragmentation is driven by both bond formation and cation stability. Upon loss of an electron, which is most commonly a non-bonding electron or lone pair, a single electron is left behind on a heteroatom. That single electron is called a radical. By stealing one electron from a neighbouring bond, that radical can become paired. A new bond forms.

For example, suppose the oxygen atom in a ketone loses an electron. That leaves the oxygen atom without an octet. The atom has seven electrons in its valence shell instead of eight. If the neighbouring carbon atom had a single electron to share, then the two atoms could each contribute an electron to form a new bond. Both atoms would have octets.
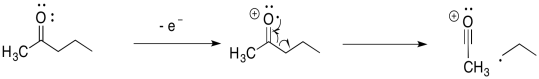
In the alpha fragmentation, a bond coming from the carbon alpha to the oxygen is broken in half. One electron remains behind, but the other electron pairs up with the radical to make a new bond.

Remember, a common term for a neighbouring atom is the alpha position. In a carbonyl, the alpha position is just the carbon next to the carbonyl. In mass spectrometry, the alpha position is the atom next to the atom that has the single electron or radical. In an alpha fragmentation, something breaks off that neighbouring atom, freeing up an electron to make a new bond. When it does so, it makes a new radical or unpaired electron; this is an example of a "propagation step" in a "radical chain reaction".
Problem MS11.1.
The alpha fragmentation shown above for 2-pentanone is just one of two such reactions. Show the other one.
Other compounds undergo similar fragmentations. Alpha fragmentations are very common in oxygen- and nitrogen-containing compounds such as alcohols and amines. Here is the pathway in 2-pentanol:
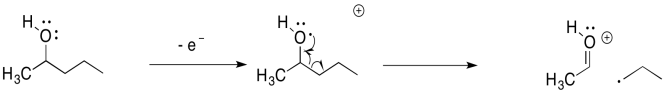
Problem MS11.2.
The alpha fragmentation shown above for 2-pentanol is just one of three such reactions for that radical cation. Show the other two.
Now, the key feature of mass spectrometry is that it can detect the mass of cations. That means that if a molecule is cleaved into two pieces, only the part with the positive charge is detected by the mass spectrometer. The other part is invisible. In the example with 2-pentanone, above, the acyl cation, CH3CO+, would be detected at m/z = 43 amu.
Problem MS11.3.
Draw the possible alpha fragmentations that would result after loss of a non-bonding electron from each of the following molecules, and indicate the m/z value of the fragments that would be detected:
a) 1-hexanol b) 3-pentanol c) 3-aminoheptane d) octanal e) 4-heptanone
McLafferty Rearrangement
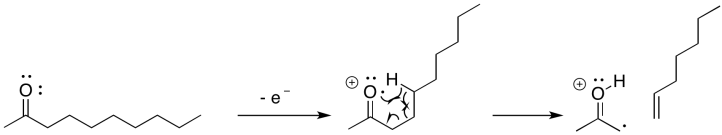
Another common pathway is called a McLafferty rearrangement. It happens in slightly longer chains. That's because it depends on a chain wrapping around until it loops; then, one end of the chain can react with another. The end result is the loss of an alkene from the original radical cation; however, unlike the alpha-fragmentation, both the cation and the radical remain in the same molecule after the Mclafferty rearrangement.
If you know anything about conformational analysis or about transition states, it might not be surprising to hear that a six-atom interaction is a key feature of this event. In the following example, involving 2-decanone, if the think of the oxygen as atom number 1, we can see a hydrogen being abstracted from atom 5; the hydrogen is the sixth atom in a row, extending from the oxygen.
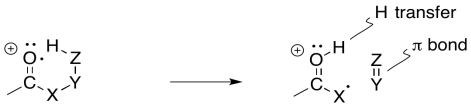
Sometimes it is helpful to break down complicated things into simpler parts. That lets us focus on one aspect of the reaction at a time. In this case, there is a hydrogen atom abstraction that occurs between the site of the initially-formed radical and a hydrogen atom that is the sixth atom along. At the same time, as that new radical forms, it begins to pull an electron out of a neighbouring bond so that it can be paired up. That aspect is like an alpha fragmentation.
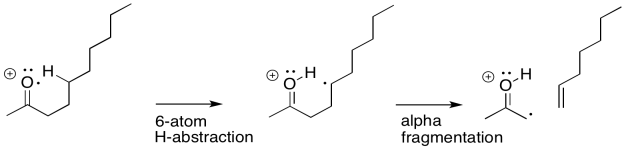
The actual reaction happens all at once, however, rather than one step at a time. As the hydrogen atom is abstracted, the remaining electron pairs up with a partner that comes from a neighbouring bond. That neighbouring bond is part of the six-membered ring transition state.
Problem MS11.4.
Provide mechanisms for McLafferty rearrangements for each of the following esters. Also, include the m/z values of the corresponding peaks in the mass spectrum.
a) 5-methylheptan-3-one
b) ethyl 2,3-dimethylbutanoate
c) isopropyl 2-methylbutanoate
Inductive Cleavage
Inductive cleavage may be the simplest mechanism of all. If an electron is lost from an electronegative atom, the simplest way to manage cation stability is to completely transfer the positive charge to a less electronegative atom.
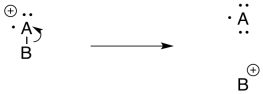
If, for example, and oxygen or a nitrogen is attached to a carbon, then once the positive charge forms on the oxygen, it can pull a pair of electrons away from the carbon.
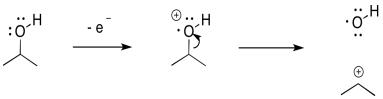
In that one, heterolytic bond cleavage, the oxygen has rid itself of the positive charge. Now it is carbon's problem. Carbon is less electronegative than oxygen, so the reaction is favoured in this direction.
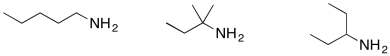
Problem MS11.5.
Order the following compounds from most likely to undergo inductive cleavage to least likely to undergo inductive cleavage.
There is an added variation of this pathway that occurs specifically with alcohols. The departing OH radical can abstract a hydrogen atom from the next position in the molecule. This step is driven by formation of an OH bond, which is relatively strong. Overall, the result is the loss of a water molecule from the molecular ion.
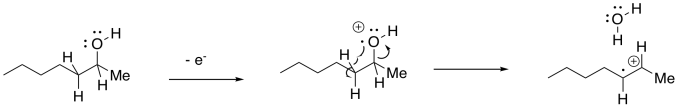
Sigma Cleavage
What if there are no obvious places for the initial ionisation to occur? A saturated hydrocarbon, for example, has no lone pairs. It doesn't even have any pi bonds. There are no clear positions where a cation would form.
Nevertheless, under ionising conditions, an electron can be knocked free from a hydrocarbon, and it has to come from a sigma bonding orbital; there isn't anywhere else. When that happens, the sigma bond has already been broken, or at least greatly weakened, in one step. We have gone from a two-electron bond to a one-electron bond; the stabilisation energy is only half what it started out as, and so the bond breaks easily.
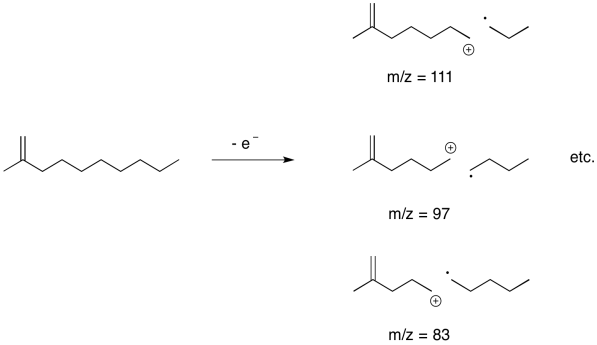
It's very common in the mass spectra of hydrocarbon chains to see a series of peaks 14 units apart. That difference corresponds to one CH2 unit in the hydrocarbon chain.
Problem MS11.6.
Predict the peaks that would be observed arising from sigma cleavage in the following compounds.
a) hexane
b) butylbenzene
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: