Reactivity in Chemistry
Mechanisms of Glycolysis
GL9. Solutions for Selected Problems.
ProblemGL1.1.
Bonds Broken:
C-C 6 x 80 kcal/mol = 480 kcal/mol
C-H 16 x 100 kcal/mol = 1,600 kcal/mol
O=O 7 x 120 kcal/mol = 840 kcal/mol
Total: 2,920 kcal/mol
Bonds Made:
C=O 14 x (- 190 kcal/mol) = - 2,660 kcal/mol
O-H 16 x (- 110 kcal/mol) = -1,760 kcal/mol
Total: -4,420 kcal/mol
Overall: 1,240 - 4,420 kcal/mol = -1,500 kcal/mol
ProblemGL1.2.
Bonds Broken:
C-C 7 x 80 kcal/mol = 560 kcal/mol
C-H 18 x 100 kcal/mol = 1,800 kcal/mol
O=O 12.5 x 120 kcal/mol = 1,500 kcal/mol
Total: 3,860 kcal/mol
Bonds Made:
C=O 16 x (- 190 kcal/mol) = - 3,040 kcal/mol
O-H 18 x (- 110 kcal/mol) = -1,980 kcal/mol
Total: -5,020 kcal/mol
Overall: 3,860 - 5,020 kcal/mol = -1,160 kcal/mol
ProblemGL1.3.
Bonds Broken:
C-C 6 x 80 kcal/mol = 480 kcal/mol
C-H 7 x 100 kcal/mol = 700 kcal/mol
C-O 7 x 85 kcal/mol = 595 kcal/mol
O-H 5 x 110 kcal/mol = 550 kcal/mol
O=O 6 x 120 kcal/mol = 840 kcal/mol
Total: 3,165 kcal/mol
Bonds Made:
C=O 12 x (- 190 kcal/mol) = - 2,280 kcal/mol
O-H 12 x (- 110 kcal/mol) = -1,320 kcal/mol
Total: -3,600 kcal/mol
Overall: 3,165 - 3,600 kcal/mol = -435 kcal/mol
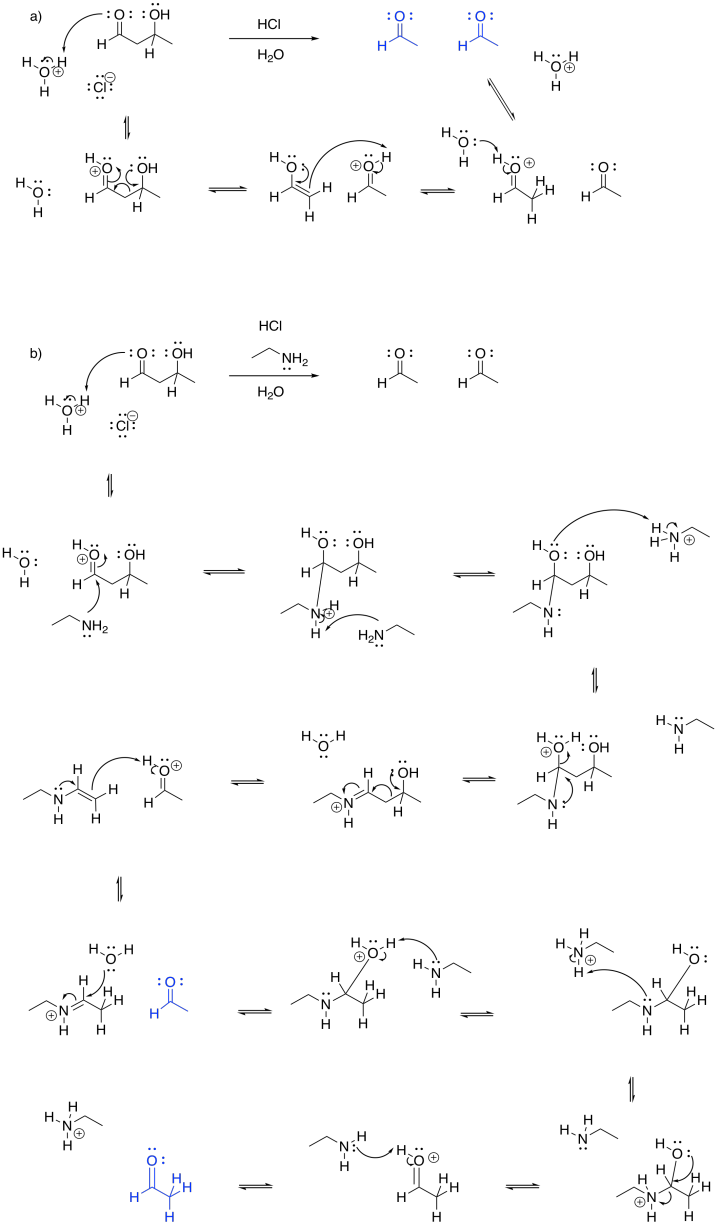
ProblemGL1.4.

Problem GL1.5.
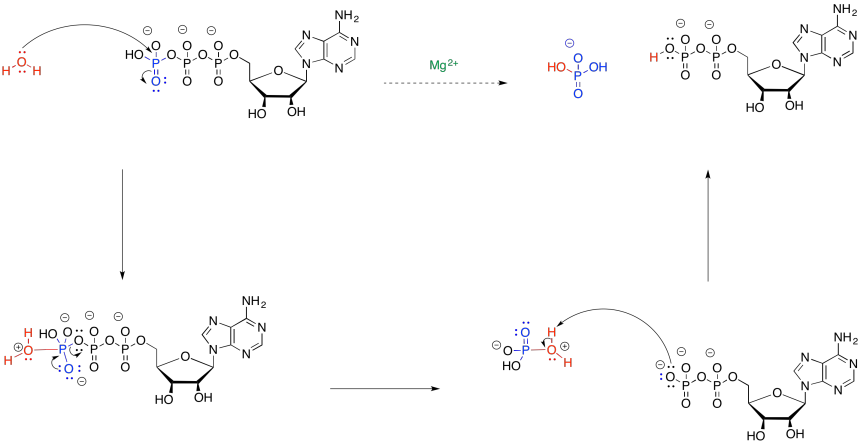
In the mechanism for hydrolysis, water acts as a nucleophile and ATP acts as an electrophile. That's a problem because ATP is negatively charged. It will not attract electrons very easily. By binding to magnesium ion (Mg2+), the charge on the ATP will be lowered, accelerating the reaction with water.
Problem GL2.1.
glucose + 2 ATP → 2 G3P + 2 ADP
Problem GL2.2.
G3P + NAD+ + PO43- + 2 ADP → pyr + NADH + 2 ATP + H2O
Problem GL2.3.
First we need to realise that one glucose gives rise to two molecules of G3P, so the second phase occurs twice for every glucose molecule consumed.
2 G3P + 2 NAD+ + 2 PO43- + 4 ADP → 2 pyr + 2 NADH + 4 ATP + 2 H2O
Adding the equations for the two phases together gives:
glucose + 2 ATP + 2 G3P + 2 NAD+ + 2 PO43- + 4 ADP → 2 G3P + 2 ADP + 2 pyr + 2 NADH + 4 ATP + 2 H2O
That equation can be simplified, because some things appear on both the left and the right. It's just like algebra.
glucose + 2 NAD+ + 2 PO43- + 2 ADP → 2 pyr + 2 NADH + 2 ATP + 2 H2O
Problem GL2.4.
Problem GL2.5.
Problem GL2.6.
Problem GL2.7.
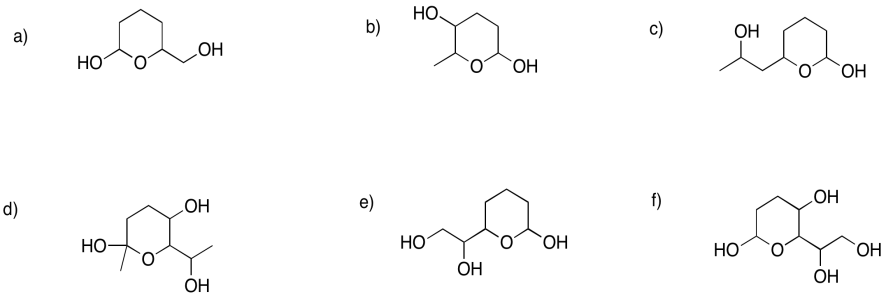
Problem GL2.8.
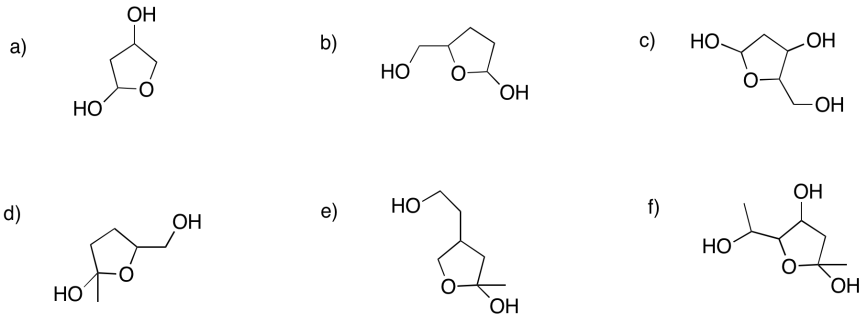
Problem GL3.1.
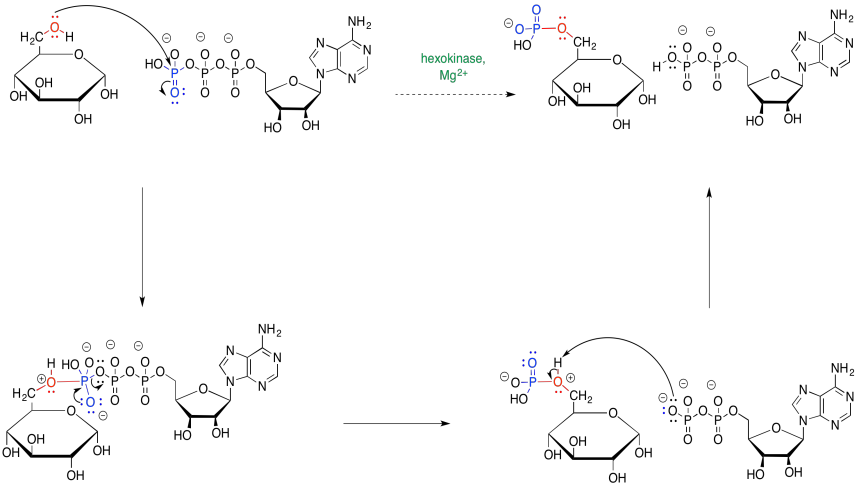
Problem GL3.2.
Problem GL3.3.
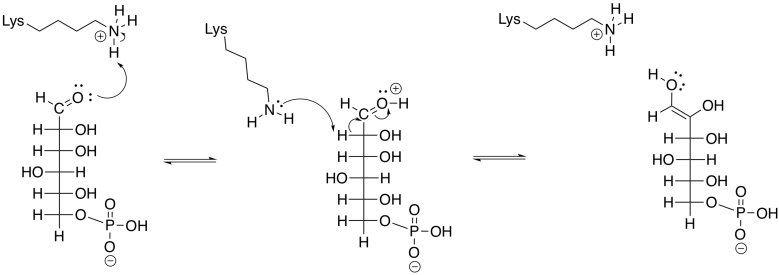
Problem GL3.4.
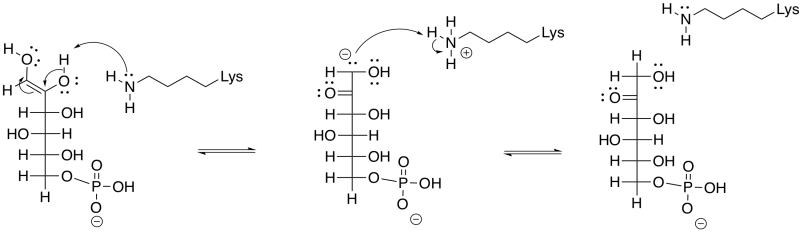
Problem GL3.5.
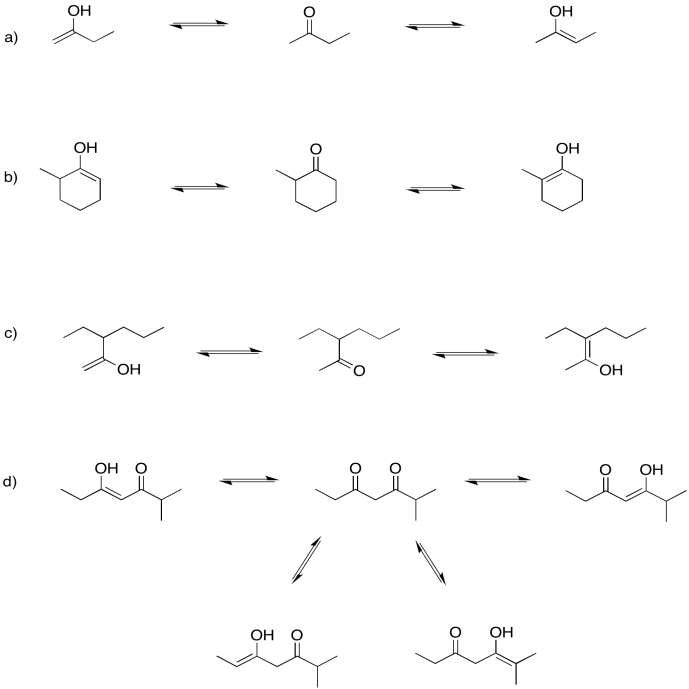
Problem GL3.6.
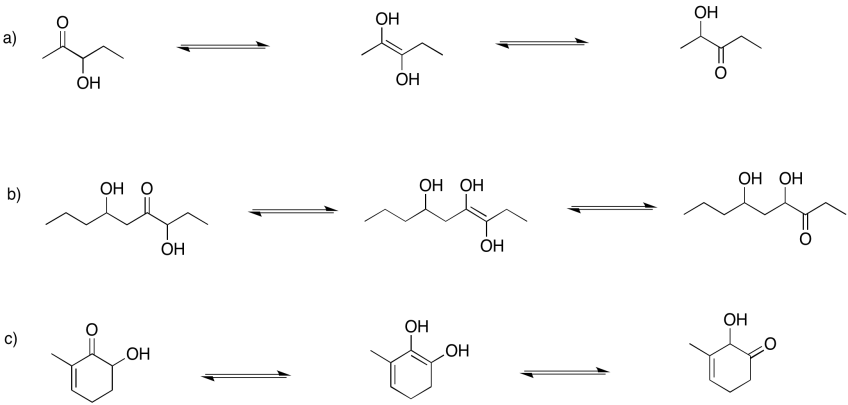
Problem GL4.1.
Problem GL4.2.
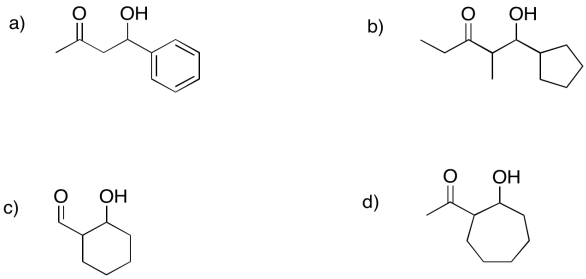
Problem GL4.3.
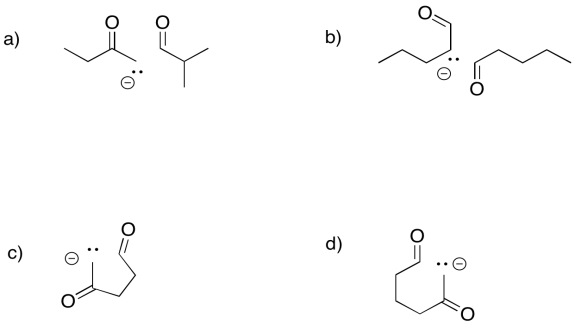
Problem GL4.4.
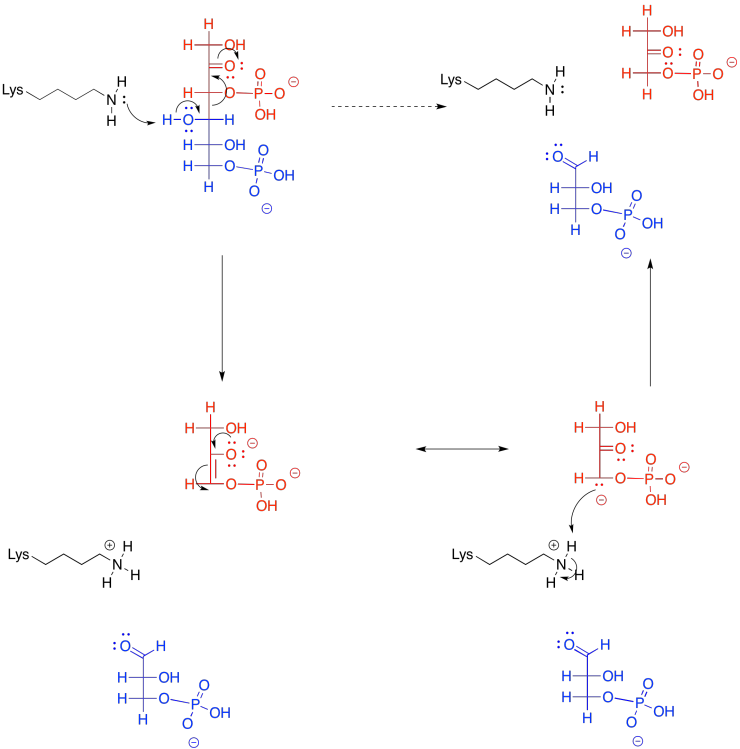
Problem GL4.5.
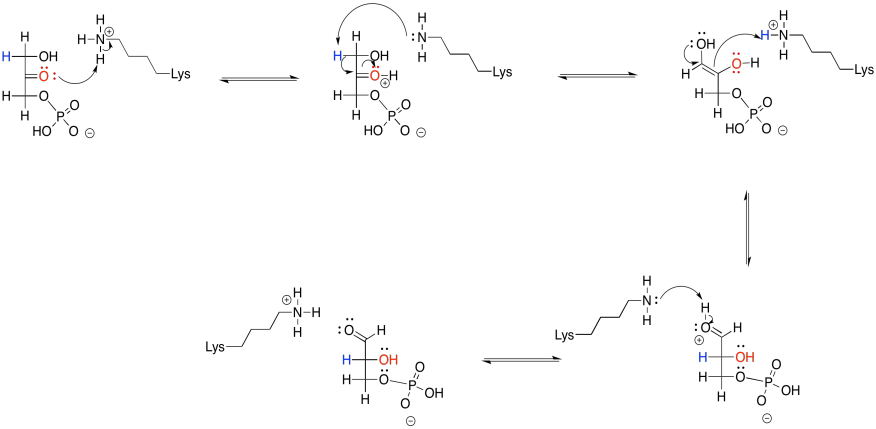
Problem GL5.1.
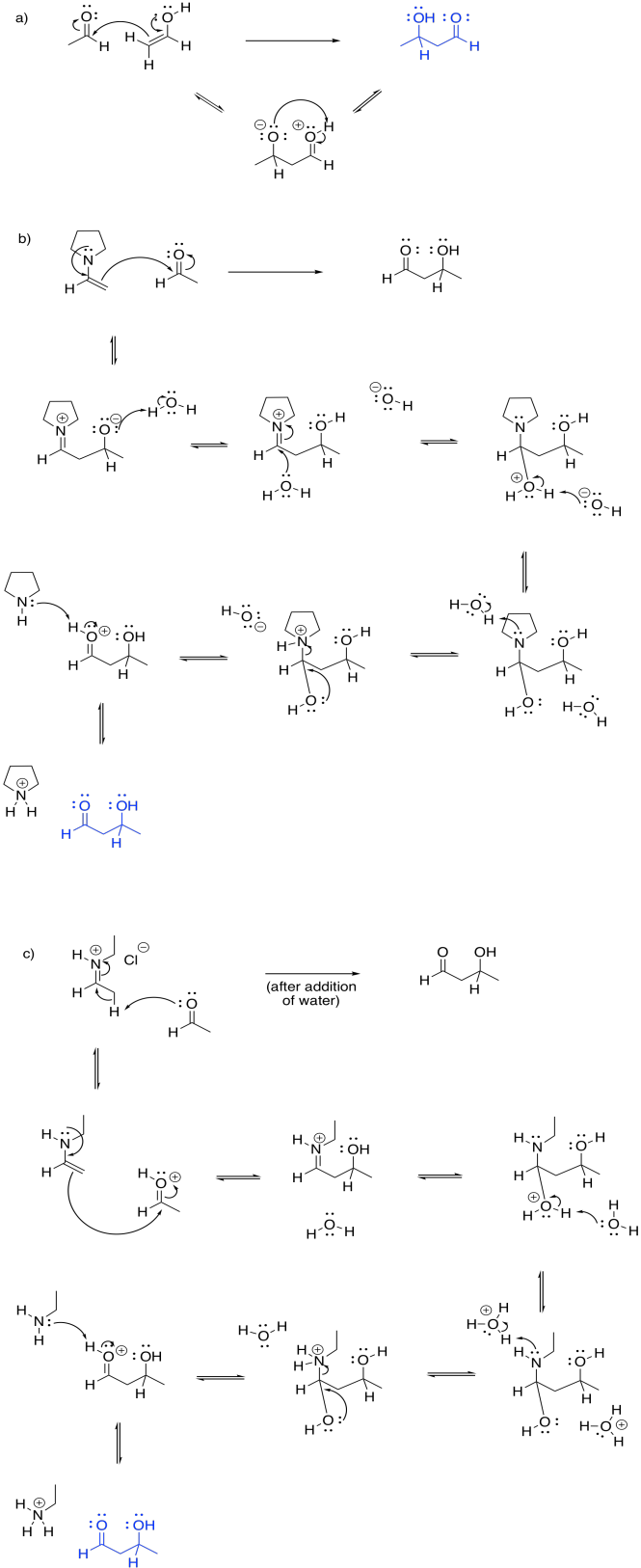
Problem GL5.2.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: