Reactivity in Chemistry
Mechanisms of Glycolysis
GL8. Thermodynamics of Glycolysis
Glycolysis is intimately linked to the release of energy in biological systems, and harnessing that energy to do work. That's what the field of thermodynamics is all about. In this section, we will take a very brief look at some of the energetic considerations of this pathway.
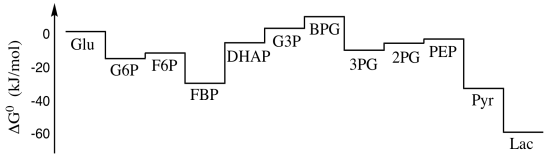
We have seen that glycolysis is a sequence of reactions leading from one intermediate compound in the pathway to the next. (To see that pathway again, click here.) Inevitably, there are energy changes associated with each of those reactions. Some of the reactions may be endothermic, others may be exothermic; some may be essentially irreversible, whereas others may occur in equilibrium. If we map these energy changes out from start to finish, we get a picture like the one below. It's a roller coaster, with lots of energetic drops but just as many hills, and it becomes difficult to think of glycolysis as a process that releases energy, except for the dramatic drop in the last couple of steps.

Figure GL8.1. Thermodynamic changes along the glycolysis pathway under standard conditions.
Where does a picture like this one come from? Well, it depicts a series of reactions, and the energy change associated with each reaction. We can determine the energy change associated with a specific reaction in a few different ways. Calorimetry may be the most straightforward way. It lets us measure the heat released or absorbed by a reaction.
A calorimeter is a well-insulated device in which we can perform a reaction. A thermometer tells us the temperature change as a result of the reaction. We can calibrate the device by releasing known amounts of heat and seeing how much its temperature rises. Consequently, we can also use that correlation backwards: given the temperature rise, we can deduce how much energy was released during a reaction.
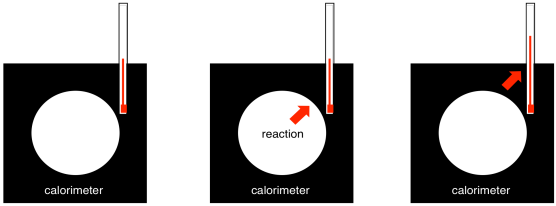
Figure GL8.2. A calorimeter measures heat changes during a reaction.
Now, if enough people have studied this sort of thing for long enough, we can begin to compile a lot of data. Given enough data, you actually might not need to perform calorimetry to determine how much energy is involved in a reaction.
To illustrate why, consider one of the most common kinds of thermodynamic data you can find: heats of formation. The heat of formation of a compound is the energy involved when the compound is formed from the elements. So, for example, the heat of formation of methane would be the energy involved when hydrogen cas combines with carbon to form methane:
2 H2 + C → CH4 ΔH = ??
It would be difficult to perform calorimetry in this case. First of all, there are just too many things that could happen if you managed to get hydrogen and carbon to combine; there are many other compounds made from hydrogen and carbon, so who knows what reaction would really occur?
But we find that heat of formation indirectly, using other data. We can burn methane:
2 O2 + CH4 → CO2 + 2 H2O ΔH = -802 kJ/mol
We can burn hydrogen to get water:
H2 + 0.5 O2 → H2O ΔH = -285.8 kJ/mol
We can burn carbon to get carbon dioxide:
O2 + C → CO2 ΔH = -393.5 kJ/mol
Well, that just seems like a series of random facts, but equations of reaction are quite a bit like algebraic equations, and those reaction arrows are quite a bit like equals signs. If we keep that in mind, we can manipulate these equations to get useful information. For example, what would happen if we took the middle reaction and multiplied it by two?
2 H2 + O2 → 2 H2O ΔH = -571.6 kJ/mol
Just as in algebra, if we multiply every term in an equation by the same factor, we end up with an equivalent equation. It's a perfectly legal operation. Note that if we multiple the equation by two, we also multiply the energy by two; it's part of the equation.
Now, you probably already know what happens if we consider one of these equations in reverse:
CO2 + 2 H2O → 2 O2 + CH4 ΔH = +802 kJ/mol
If the reaction is exothermic in one direction, then it must be endothermic in the other. One way is downhill, so the other way is uphill.
Look what happens if we add these three reactions together in their current forms:
CO2 + 2 H2O → 2 O2 + CH4 ΔH = +890.3 kJ/mol
2 H2 + O2 → 2 H2O ΔH = -571.6 kJ/mol
O2 + C → CO2 ΔH = -393.5 kJ/mol
CO2 + 2 H2O + 2 O2 + 2 H2 + C → 2 O2 + CH4 + 2 H2O + CO2 ΔH = +802 kJ/mol
Several things cancel on the left and right, leaving:
2 H2 + C → CH4 ΔH = -74.8 kJ/mol
What that means is that, if we have energetic information about some reactions, and we can combine the equations for those reactions to get a new equation of reaction, then we automatically get the energy associated with that new reaction.
Essentially, if we want to know about the energetics of producing methane from carbon and hydrogen, then it doesn't matter how we get from the carbon and the hydrogen to the methane. We can first take the carbon and combine it with oxygen, not hydrogen, and make carbon dioxide. Then, we can take hydrogen and combine it with oxygen, not carbon, to make water. If, finally, we combine the water and the carbon dioxide we have made and produce methane, then the energy of that whole, roundabout process is the same as if we converted the carbon and the hydrogen directly into methane.
This idea illustrates something called Hess' Law. The overall energy required to get from one set of reactants to another set of products is always the same, regardless of the path taken. Hess' Law is true because energy is a "state function". If we know the state that something is currently in - for example, methane in the gas phase at a certain temperature and pressure - then we know its energy. It doesn't matter what has happened to it before, or how it got to its current state.
So, we can measure the heat of a reaction using a calorimeter. We can use Hess' Law to deduce what the heat of a reaction must be, based on the heats of other reactions. We can also infer the free energy of an equilibrium reaction based on the equilibrium constant for the reaction. To do that, we make use of the mathematical relationship between the standard free energy of a reaction, ΔG0, and the equilibrium constant, K.
in which R is the ideal gas constant, 8.314 J K-1 mol-1, and T is the temperature in Kelvin.
Conceptually, if we know how strongly an equilibrium leans in the forward or reverse direction, we can calculate the energy difference between the product side an the reactant side. The larger the equilibrium constant, the more exothermic (or exoergonic) the reaction is. That means it's just a matter of measuring the concentrations of reactants and products at equilibrium and then calculating the free energy change.
In the next section, we are going to see a slightly different picture of the energetic terrain of glycolysis. A slightly different picture comes about because of the different concentrations of all of these compounds in the cell.
See the section on metabolic pathways at Henry Jakubowski's Biochemistry Online.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: