Reactivity in Chemistry
Coordination Chemistry
CC7. Hard & Soft Acid & Base Concepts
Not all metals form coordination complexes with all possible ligands. Some metals are more likely to form compounds with certain ligands. This observation has eventually led to a classification system called Hard and Soft Acids and Bases (HSAB).
In a nutshell, smaller or more highly charged metal ions are called hard acids. They are more likely to bind to hard bases, which typically have small donor atoms such as oxygen or nitrogen.
Typical hard acids are titanium(IV), tantalum(V), magnesium(II) and lithium(I). Oxide, hydroxide and carbonate (CO32-) are some typical hard bases.
Larger, more polarizable metal ions with lower charges are called soft acids. "Polarizable" means they have large, easily distorted clouds of electrons. They are more likely to bind to soft bases, which are typically large anions such as sulfide or selenide.
"hard" acids are small or highly charged
"soft" acids are larger or more polarizable or have lower charge
"hard" bases contain smaller, less polarizable donor atoms, usually oxygen or nitrogen
"soft" bases contain larger, more polarizable donor atoms, such as sulfur or phosphorus
hard ions tend to bind well together; soft ions tend to bind well together
Problem CC7.1.
Suggest which of the following ions is harder.
a) zinc(II) or mercury(II) b) potassium(I) or copper(I) c) iron(II) or iron(III)
Problem CC7.2.
Suggest which of the following bases is softer.
a) Me3P or Me3N b) chloride or iodide c) amide (NH2-) or azide (N3-)
There are some obvious HSAB applications in metallurgy and geology. Some common minerals of hard metals are rutile (titanium oxide, TiO2), dolomite (magnesium and calcium carbonate CaMg(CO3)2) and chromite (iron chromium oxide, FeCrO4). Fluoride, carbonates, oxides, phosphates and sulfates are examples of hard bases.
Some prevalent minerals of soft metals are galena (lead sulfide, PbS2) and cinnabar (mercury sulfide, HgS). Sulfides are the most common soft bases in geology, although the larger halides, like bromide and iodide, are also soft.
Some metals can pair with either hard or soft bases, particularly those metals from the middle of the transition metal group. For example, iron(III) is often found as hematite (iron oxide, Fe2O3), whereas iron(II) can also be found as pyrite (iron sulfide, FeS). Molybdenum(VI) can be found as powellite (calcium molybdenum oxide, CaMoO4), but the most commonly mined ore contains molybdenum(IV), found in molybdenite (MoS2).
Problem CC7.3.
Propose a formula for a plausible mineral containing each of the following ions.
a) zirconium(IV) b) cadmium(II) c) tungsten(VI)
d) zinc(II) e) copper(I)
In biology, metals display aspects of hard & soft acid & base chemistry. Relatively hard potassium ions bind to oxygen atoms in DNA to help stabilize the helix structure. Calmodulin, used to aid in calcium uptake, uses hard oxygen donors in aspartate and glutamate to bind to the Ca2+.
On the other hand, copper(I) is a soft acid. In poplar plastocyanin, which aids in transferring electrons during reactions in the plant cell, the copper ion is coordinated to two nitrogen-donating histidines and two sulfur donors, a cysteine and a methionine.
Many biologically important metal ions fall under the "borderline" category between hard and soft. Iron is one of the most abundant elements on earth, and many iron compounds play important roles in biology. Many biological compounds contain iron(II), which is able to bind well to both hard and soft ligands. Consequently, it is found with anionic oxygen carboxylate donors in methane monooxygenase, neutral and anionic nitrogen porphyrin donors in heme proteins, and sulfur cysteines and sulfides in ferridoxins and other iron-sulfur clusters.
Hard and soft acid and base phenomena have been studied using molecular orbital theory and other quantitative approaches. In MO theory, it has been shown that interactions between hard anions and cations are characterized by large HOMO-LUMO separations, whereas interactions between soft anions and cations are characterized by small HOMO-LUMO separations. In other words, hard acid-base interactions are dominated by more strongly ionic character, but soft acid-base interactions are dominated by more strongly covalent character.
Problem CC7.4.<
Mercury ions, Hg(I) and Hg(II), are particularly poisonous. They can displace other metals from enzymes, so that the enzymes stop working.
a) are these ions hard or soft?
b) what amino acid residues would most likely bind to them?
Problem CC7.5.
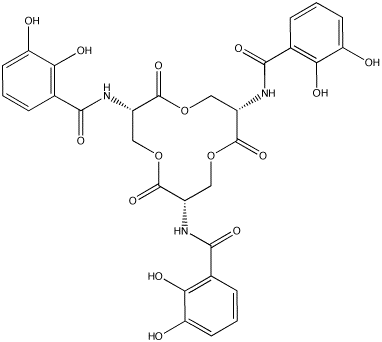
Enterobactin (below) is a molecule used by certain bacteria to bind iron(III) and transport it into the cell. The formation constant for the iron(III)-enterobactin complex is about 1049. Provide reasons why the formation constant is so high.

This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Coordination Chemistry Index
Back to Web Materials on Structure & Reactivity in Chemistry