Reactivity in Chemistry
Coordination Chemistry
CC11. Ligand Lability
Lability refers to the ease with which ligands are replaced in coordination complexes. Scandium is referred to as "labile" in the following example.
[Sc(OH2)6]Cl3
+ 6 NaSCN → Na3[Sc(SCN)6] + 3 NaCl (very fast!)
Lability refers to how easily metal-ligand bonds are broken. A compound in which metal-ligand bonds are easily broken is refered to as "labile". A compound in which metal-ligand bonds are more difficult to break is referred to as "inert".
Henry Taube (Nobel
Prize, 1983) tried to understand lability by comparing the factors that govern
bond strengths in ionic complexes to observations about the rates of reaction of
coordination complexes. He saw some things that were unsurprising.
He also drew some new conclusions based on ligand field theory.
Problem CC11.1.
In which compound from each pair
would you expect the strongest ionic bonds? Why?
a) LiF vs KBr
b) CaCl2 vs. KCl
Taube
observed that many M+1 ions (M = metal) are more labile than many M+3
ions, in general. That isn't too surprising, since metal ions
function as electrophiles or Lewis acids and ligands function as nucleophiles or
Lewis bases in forming coordination complexes. In other words, metals with
higher charges ought to be stronger Lewis acids, and so they should bind ligands
more tightly.
However, there were exceptions to that general
rule. For example, Taube also observed that Mo(V) compounds are more
labile than Mo(III) compounds. That means there is more going on here than just
charge effects.
Another factor that governs ionic bond
strengths is the size of the ion. Typically, ions with smaller
atomic radii form stronger bonds than ions with larger radii. Taube
observed that Al3+, V3+, Fe3+ and Ga3+
ions are all about the same size. All these ions exchange ligands at about
the same rate. That isn't surprising, because they have the same
charge and the same radius.
However, Cr3+ is also about the same size as those ions and it also has the same charge, but it is much less labile. Once again, there are exceptions to our regular expectations based on simple electrostatic considerations.
Furthermore, second- and third-row transition metals (Y-Cd and Ac-Hg) are much more inert than first-row transition metals (Sc-Zn). That is a little surprising, since those lower metals are much larger than the first row metals.
However, it
gives us a clue about other factors that are playing a role in lability.
In ligand field theory, second- and third-row metals have much larger d orbital
splitting energies than do first-row metals. That is sometimes explained
in terms of diffuse orbitals on these larger atoms forming stronger bonds to
ligands, and reminds us that we are not just dealing with electrostatic
interactions.
Taube wondered whether d electron
configuration influenced whether a compound is labile or inert. That idea forms
the basis of Taube's rules about lability.
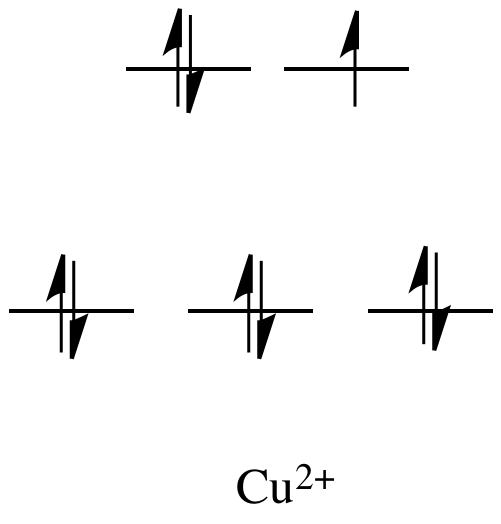
For example, metals like Ni2+ and
Cu2+ are very labile. The d orbital splitting diagrams for those compounds would have
d electrons in the eg set. Remember, the eg set arises from
interaction with the ligand donor orbitals; this set corresponds to a σ
antibonding level.

Figure CC11.1. The d orbital diagram for an octahedral Cu2+ ion.
By comparison, V2+
is rather inert. The d orbital splitting diagram in this case has
electrons in the t2g set, but none in the eg set.
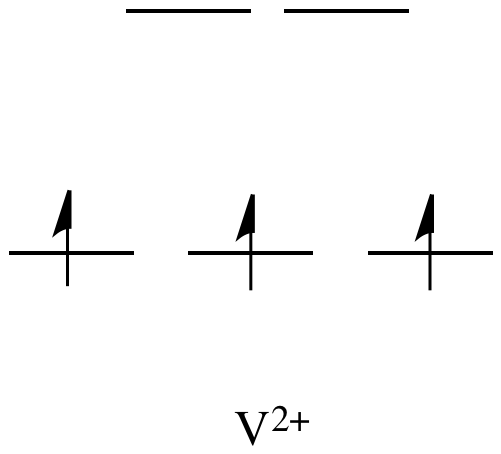
Figure CC11.2. The d orbital diagram for an octahedral V2+ ion.
So, having electrons in the higher energy, antibonding eg level weakens the bond to the ligand, so the ligand can be replaced more easily. In the absence of those higher energy electrons, the bond to the ligand is stronger, and the ligand isn't replaced as easily.
On the other hand, the Ti2+ ion doesn't have any electrons in the eg level, but it is lable like Cu2+, not inert like V2+.

Figure CC11.3. The d orbital diagram for an octahedral Ti2+ ion.
Going even further, metals like Ca2+, Sc3+ and Ti4+ are also pretty labile. The d orbital splitting diagrams in those cases are pretty simple: there are no d electrons at all in these ions.
The general observation is that having no electrons in these mostly non-bonding levels leaves the complex susceptible to ligand replacement. But it's hard to see why population of an orbital that is mostly non-bonding would have an effect on ligand bond strength.
Instead, this factor probably has something to do with the part of ligand substitution that we have ignored so far. Not only does one ligand need to leave, but a second one needs to bond in its place. So, having an empty orbital for the ligand to donate electrons into (or, put another way, not having electrons in the way that may complicate donation from the ligand) makes that part of the reaction easier.
Problem CC11.2.
Some
metals, like Mn2+, can be either labile or inert, depending on
whether they are high spin or low spin. Explain why using d orbital
splitting diagrams.
Problem CC11.3.
Predict
whether the following metals, in octahedral complexes, are labile or not.
a) Co3+ (high spin)
b) Co3+ (low spin)
c) Fe2+ (low spin)
d) Fe2+ (high spin)
e) Zn2+
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Coordination Chemistry Index
Back to Web Materials on Structure & Reactivity in Chemistry