Electrophilic Addition to Alkenes
EA9. Cyclopropanation
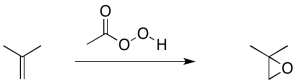
Earlier, we saw that alkenes can donate their pi electrons to oxygen electrophiles in peroxides. The result is transfer of an oxygen atom from the peroxide to the alkene. An epoxide or oxirane ring is formed.

Figure EA9.1. An epoxidation reaction.
Another, related example is cyclopropanation of an alkene. In alkene cyclopropanation, an alkene is converted into a cyclopropane. A carbon atom is donated to the alkene. Sometimes, a CH2 group is added to the double bond. Sometimes, it is another divalent carbon group, such as CCl2.
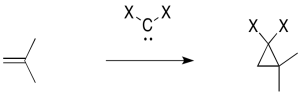
Figure EA9.2. A general cyclopropanation reaction. X is often a hydrogen or a halogen.
The addition of dichlorocarbene to an alkene a pretty common example. The dichlorocarbene is generated by treating chloroform, CHCl3, with sodium hydroxide. The sodium hydroxide abstracts a proton from the chloroform, resulting in the elimination of a chloride ion. That leaves behind a dichlorocarbene, CCl2.
CHCl3 + NaOH → CCl2 + H2O + NaCl
The dichlorocarbene adds across the double bond of an alkene to make a dichlorocyclopropane.
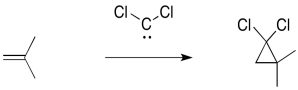
Figure EA9.3. Cyclopropanation with dichlorocarbene.
Carbenes are electrophiles because the carbon does not have an octet. The carbon has only two bonds and one lone pair. That's just three electrons, not eight. The carbon has an ampty p orbital that can accept a pair of electrons from a nucleophile. On the other hand, there is a lone pair. The carbene can be nucleophilic, too.
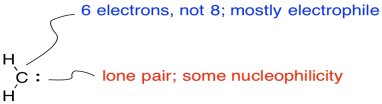
Figure EA9.4. The Lewis structure of a simple carbene.
As a result, a cyclopropanation reaction involves two bond-making events at the same time. The reaction includes the addition of the nucleophilic alkene to an electrophilic carbene's empty p orbital. At the same time, that lone pair can donate back, so that a carbocation does not actually form. That situation is similar to the interaction of a nucelophilic alkene with a bromine or mercury(II) electrophile.
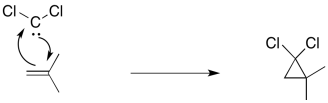
Figure EA9.5. The cyclopropanation mechanism.
Now, let's take another look at where the carbene comes from in the first place. The carbene is sometimes formed through an unusual "alpha"-elimination in the presence of strong base. Strong bases are often alkyllithium reagents, such as CH3CH2CH2CH2Li or BuLi, but KOH will work with some compounds.
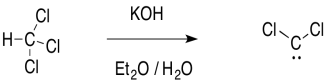
Figure EA9.7. Reaction conditions for dichlorocarbene formation.
The reaction is unusual because a proton is abstracted from one carbon atom, and a leaving group departs from the same atom. It is much more common to see the leaving group depart from the next atom over, in a beta-elimination. A beta-elimination forms an alkene from a haloalkane. An alpha-elimination forms a reactive carbene, instead.
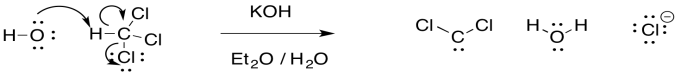
Figure EA9.7. The mechanism of dichlorocarbene formation.
That's not the only way to generate a carbene. Carbenes are frequently formed from diazo compounds. A diazo group in an NN+ group, containing an N-N triple bond. The simplest example of these compounds is diazomethane, CH2N2. Diazomethane can be used to add a simple CH2 group across an alkene double bond, forming a regular cyclopropane
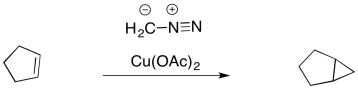
Figure EA9.8. A metal-catalyzed cyclopropanation with diazomethane.
In diazo compounds, an N2 group is hanging on by a thread. It can easily leave, resulting in a carbene. This decomposition reaction is often promoted by metal catalysts, such as copper (II) salts.
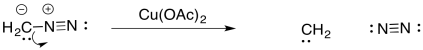
Figure EA9.9. Decomposition of diazomethane to generate a carbene.
The carbene, CH2, is even less stable than CCl2. However, in the presence of metal salts, it can be stabilized as a metal carbene complex.
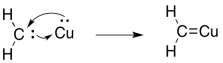
Figure EA9.10. Formation of a metal carbene or alkylidene complex.
Problem EA9.1.
Why is a carbene more stable with chlorine atoms attached?
Problem EA9.2.
Some metal carbene complexes, such as the Fischer carbene shown below, are particularly stable. Explain why.

Problem EA9.3.
Free carbenes, those not attached to a metal ion, are typically so unstable that they can only be generated briefly in solution before they react with a nucleophile, such as an alkene. Arduengo carbenes, such as the one below, are stable enough to be put in a bottle and stored in the refrigerator. Explain why.
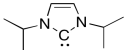
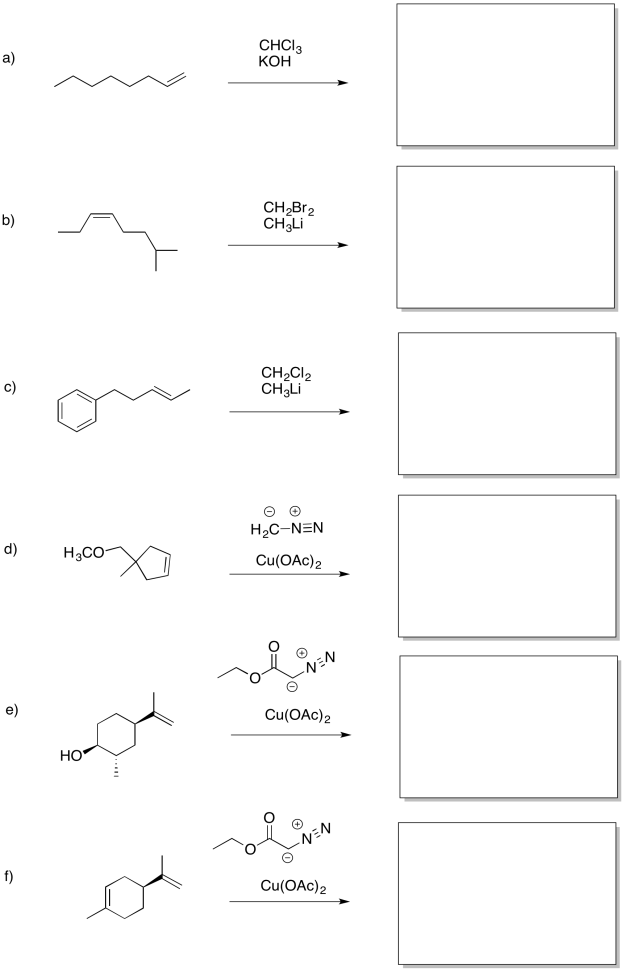
Problem EA9.4.
Predict the products of the following reactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: