Reactivity in Chemistry
Electrophilic Addition to Alkenes
EA2. Cations in Electrophilic Addition
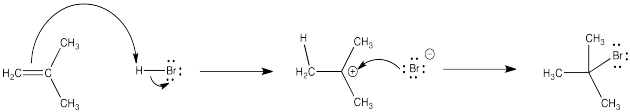
Many of the reactions of alkenes begin with a protonation step. The cation that forms then undergoes a second step in which it combines with the counterion from the acid.

Figure EA2.1. Mechanism of addition of HBr across an alkene double bond.
In the first step, the alkene's π bond is the nucleophile and the proton is the electrophile. In the second step, the bromide is the nucleophile and the cation is the electrophile.
If you are familiar with nucleophilic aliphatic substitution, you will already know that the presence of a cationic intermediate signals some potential complications in this reaction.
One issue is the problem of stereochemical control. A carbocation is trigonal planar, because the carbon with the positive charge has only three groups attached to it. Because the cation is trigonal planar, the bromide ion that combines with it can approach from either side. It can come from above or below the trigonal plane.
That fact may have no effect whatsoever. However, if the alkene (and the cation it forms) is prochiral, meaning it has the potential to form a new chiral center during this reaction, then there is a choice of which enantiomer to make.
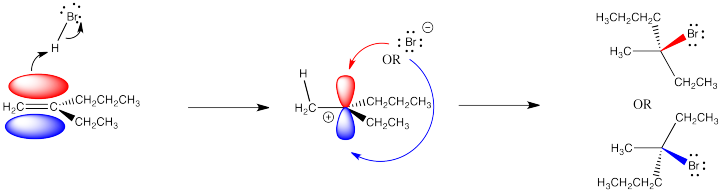
Figure EA2.2. Prochirality: addition of HBr across an alkene double bond can form a new chiral centre.
A prochiral carbocation is easy to recognize because the cationic carbon has three different groups attached to it. The fourth group added, the nucleophile, would result in four different groups attached to that carbon, making it a chiral center. In order to recognize a prochiral alkene, you can picture what the alkene would look like after the reaction has taken place: will there be four different groups?
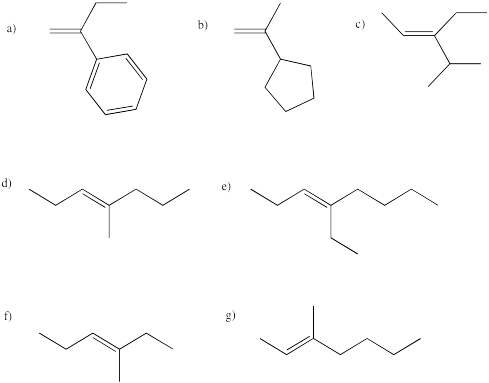
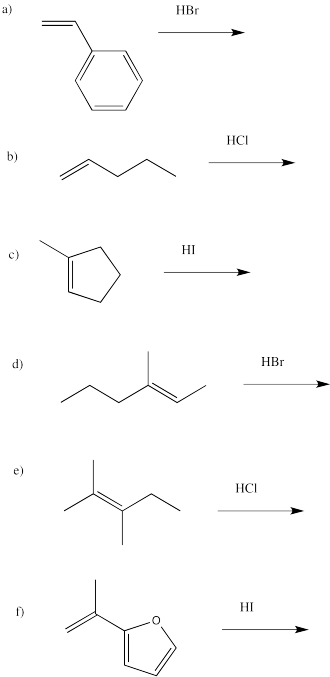
Problem EA2.1.
Which of the following alkenes are prochiral?
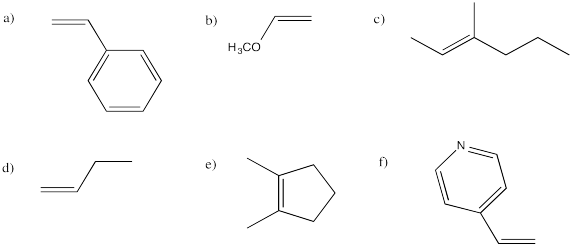
Problem EA2.2.
Addition of the nucleophile to one face of the alkene will result in a stereocentre with R configuration. That face is called the re face. Adding it to the other will lead to formation of S configuration. That face is called the si face.
In the following alkenes, identify whether we are looking at the re face or the si face in terms of the product we would get through addition of HBr..
Problem EA2.3.
Draw the products of the following reactions, paying attention to stereochemistry.
In addition to the problem of stereochemistry, electrophilic additions of alkenes also present potential regiochemical complications. As in aliphatic nucleophilic substitutions, formation of a cation often opens the door to rapid rearrangement via 1,2-hydride shifts. There may be one hydride shift or there may be many of them in a row. For example, reaction of 1-hexene under acidic conditions leads to addition products at the third carbon in the chain as well as the second.
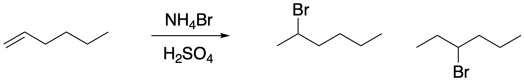
Figure EA2.3. Rearrangement during the addition of HBr across an alkene double bond.
That second product occurs because of a 1,2-hydride shift in the cation intermediate.
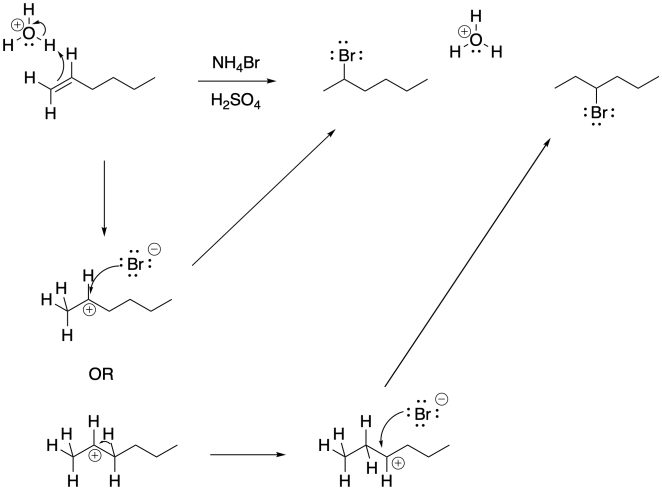
Figure EA2.4. The mechanism of rearrangement involves a 1,2-hydride shift.
These hydride shifts happen pretty easily. Overlap of a hydrogen atom with the empty p orbital of the the adjacent cation leads to a short hop from one carbon to the next.
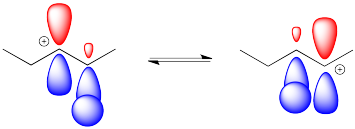
Figure EA2.5. Orbital overlap in a hydride shift.
A hydride shift from one secondary carbon to the next, as illustrated in the above example, is thermodynamically pretty neutral. Because the barrier is low, it happens quickly, but there isn't a driving force fo the hydride to shift one way or the other. Instead, both cations result. There is a mixture.
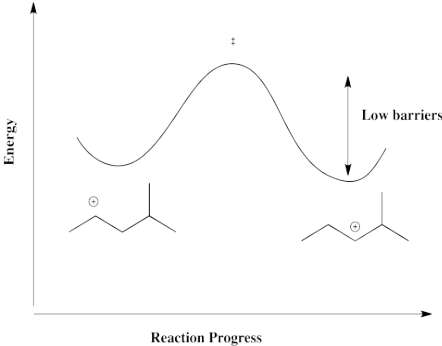
Figure EA2.6. Reaction progress diagram between two secondary carbocations.
However, in a case in which the cation can form in a more stable position, such as a tertary position, there is a driving force for the reaction to go one way. The barrier would be too high for it to get back.
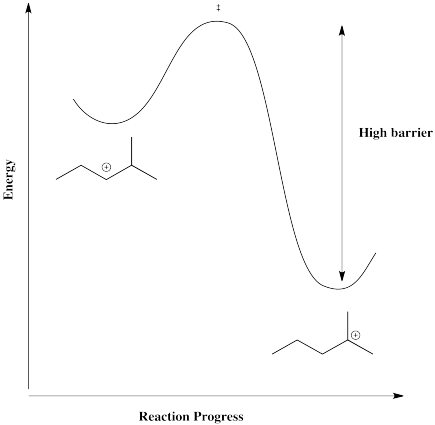
Figure EA2.7. Reaction progress diagram between a secondary and a tertiary carbocation.
As a result, when the counterion combines with the cation, it may do so in a position away from the original double bond. Furthermore, these 1,2-shifts in cations are not restricted to hydrides. Alkyls and aryls (phenyls) are also known to rapidly undergo 1,2-shifts, especially if a more stable cation results.
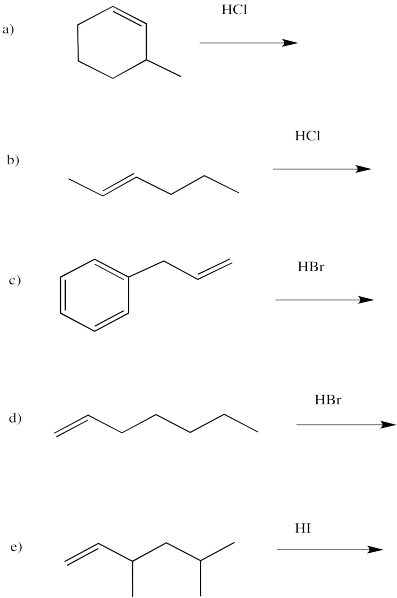
Problem EA2.4.
Draw the products of the following reactions, paying attention to regiochemistry.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: