Reactivity in Chemistry
Electrophilic Addition to Alkenes
EA14. Solutions for Selected Problems.
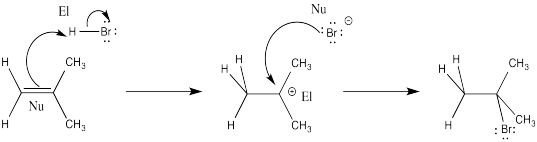
Problem EA1.1.
El = electrophile; Nu = nucleophile

Problem EA1.2.
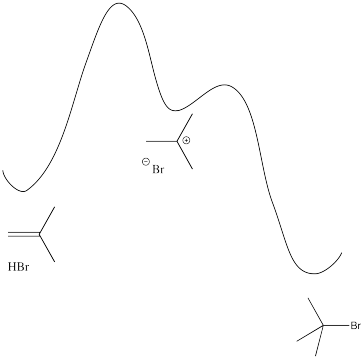
Problem EA1.3.
Rate = d[alkyl bromide] / dt = k [alkene] [ HBr]
Problem EA1.4.
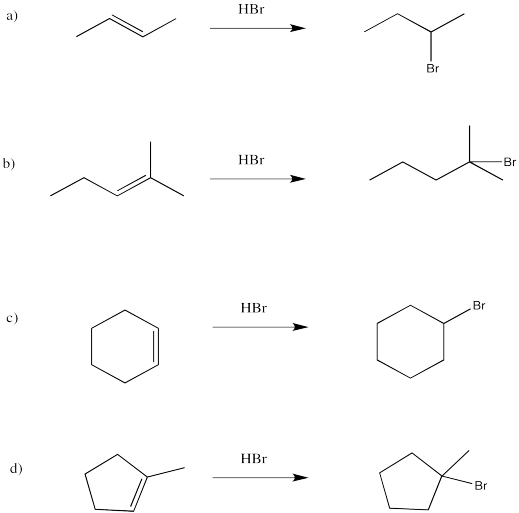
Problem EA2.1.
a, e, f, g are prochiral.
Problem EA2.1.
a) re b) si c) re d) si e) either; if the Br adds on one end of the double dond it is re, but at the other it is si f) re
Problem EA2.3.
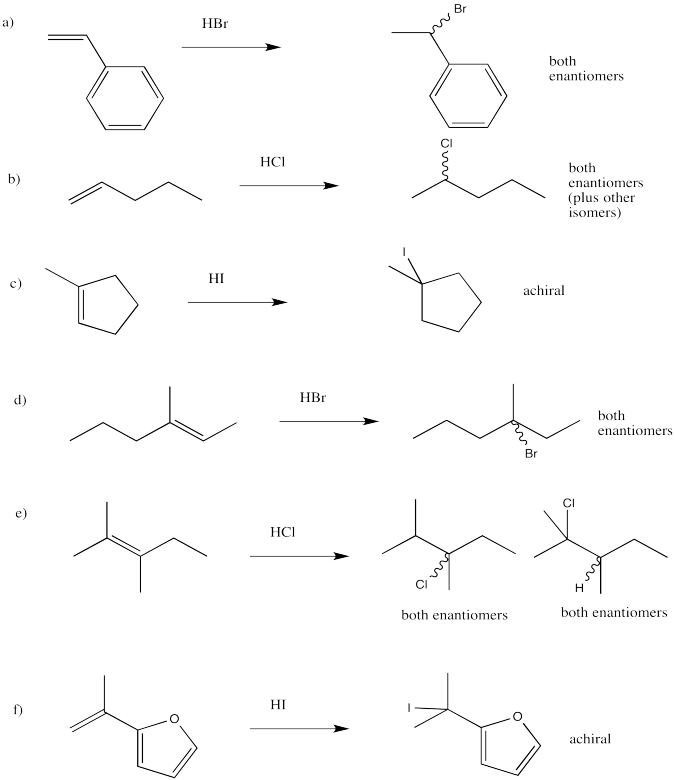
Problem EA2.4.
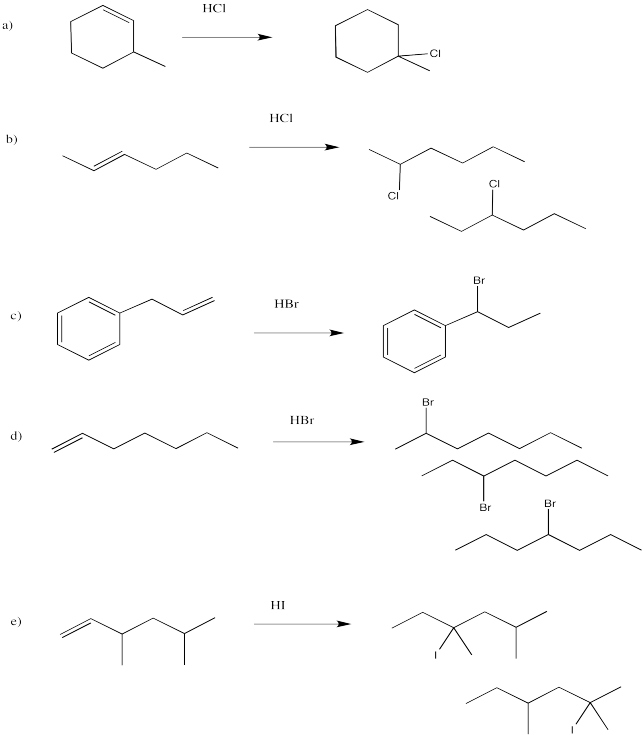
Problem EA3.1.
If the acid is regenerated at the end of the reaction, it isn't a reagent. It is a catalyst. It makes addition of water to the double bond occur much more quickly than if water acted alone, since water would never manage to protonate the alkene.
Problem EA3.2.
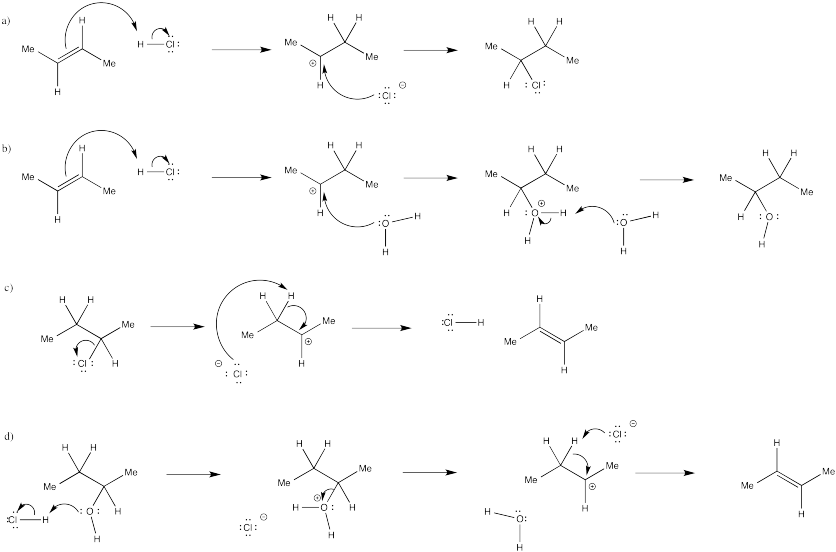
Problem EA3.3.
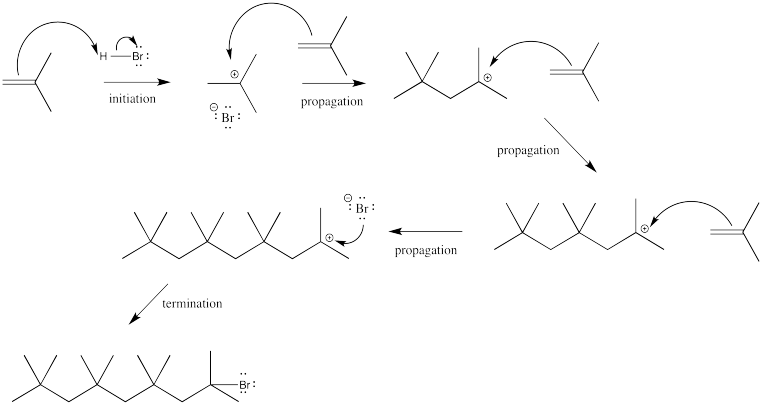
Problem EA4.1.
Two products are formed and they are enantiomers.
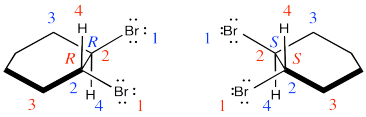
Problem EA4.2.
They are diastereomers. One chiral center has the same configuration in both compounds but the others are opposite.
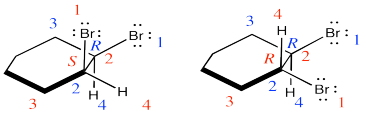
Problem EA4.3.
The second bromine could occupy any of the secondary positions if there were a true carbocation. That doesn't happen; the second bromine occupies only the position next to the other bromine.
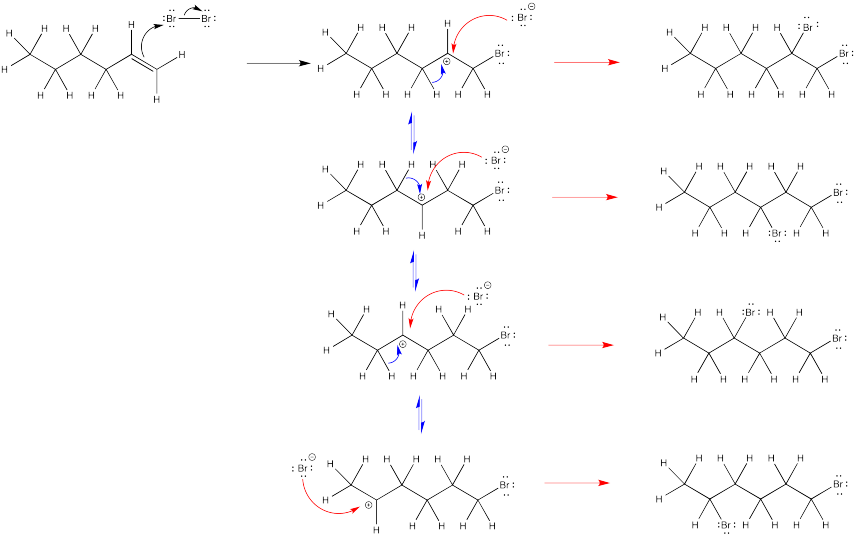
Problem EA4.5.
The nucleophile in the second step changes under different conditions.
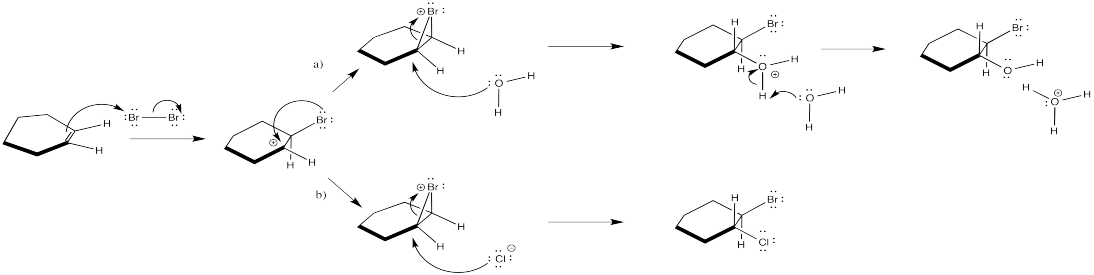
Problem EA4.6.
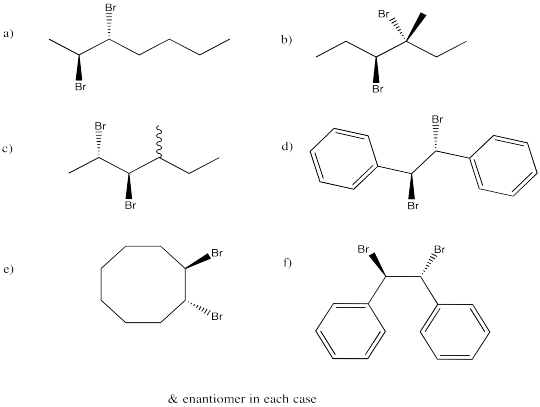
Problem EA4.7.
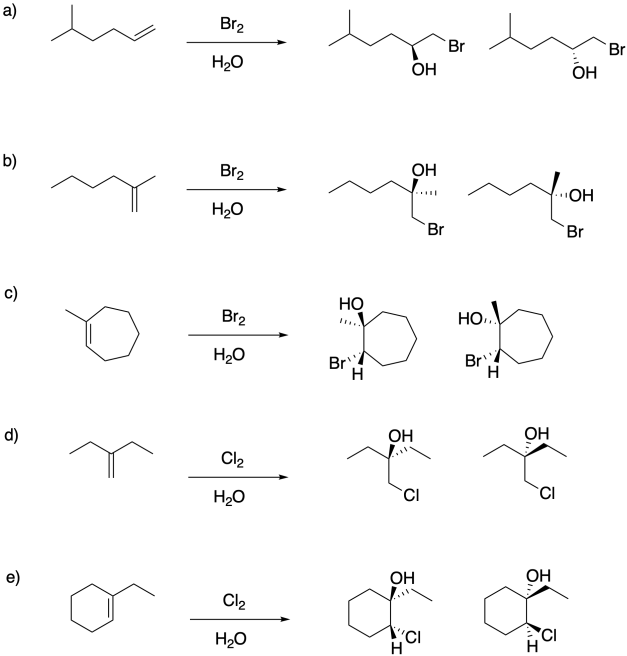
Problem EA5.1.
Problem EA5.2.
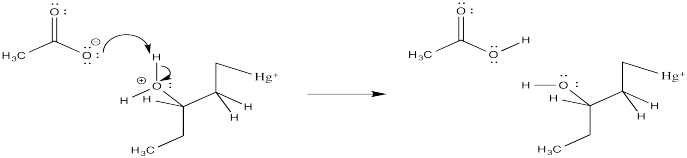
Problem EA5.3.
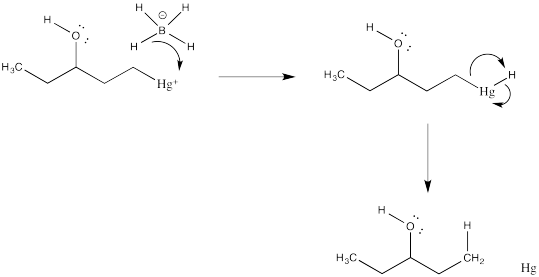
Problem EA5.4.
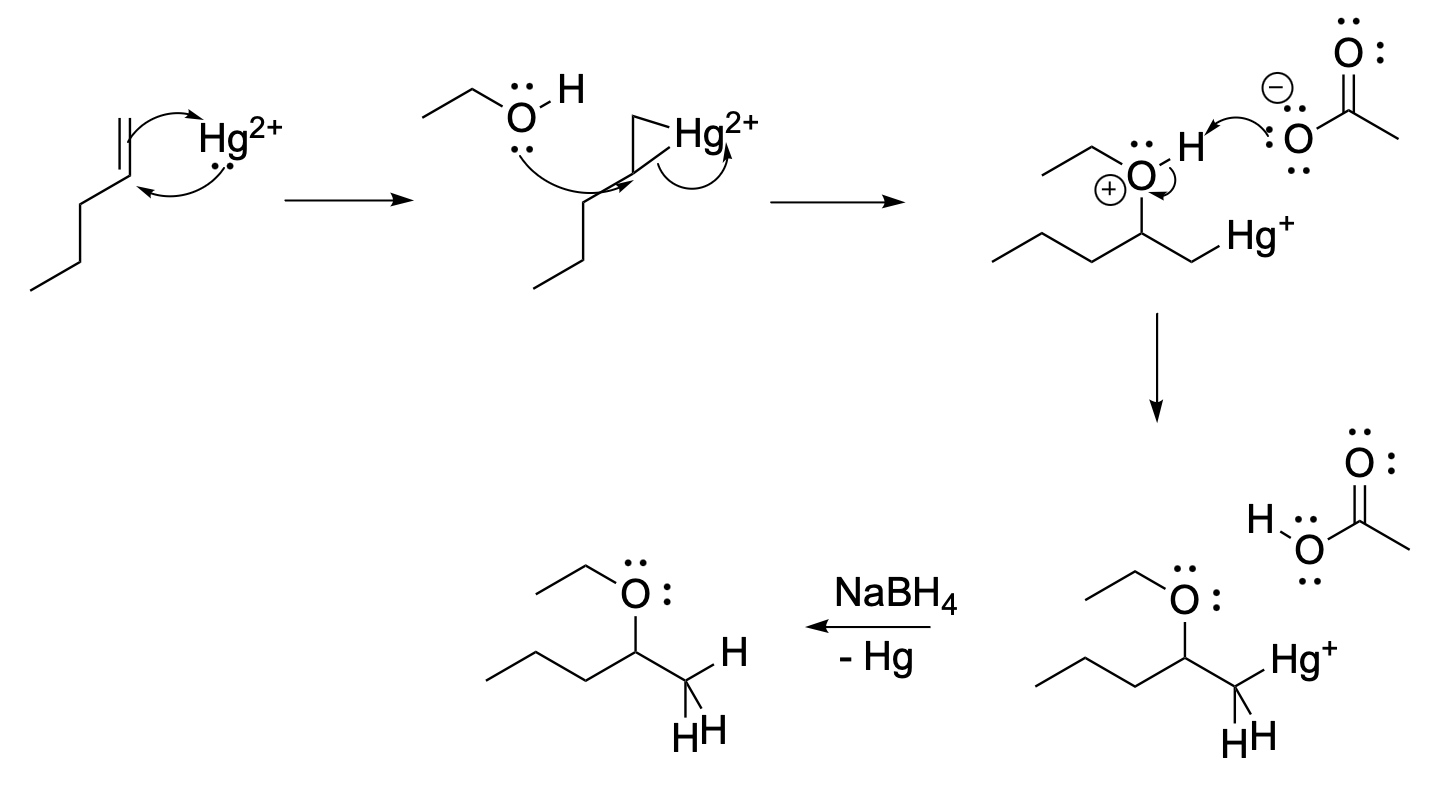
Problem EA5.5.
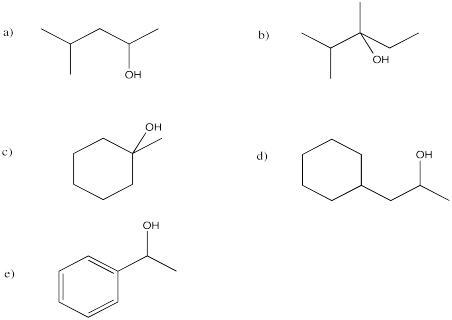
Problem EA5.6.
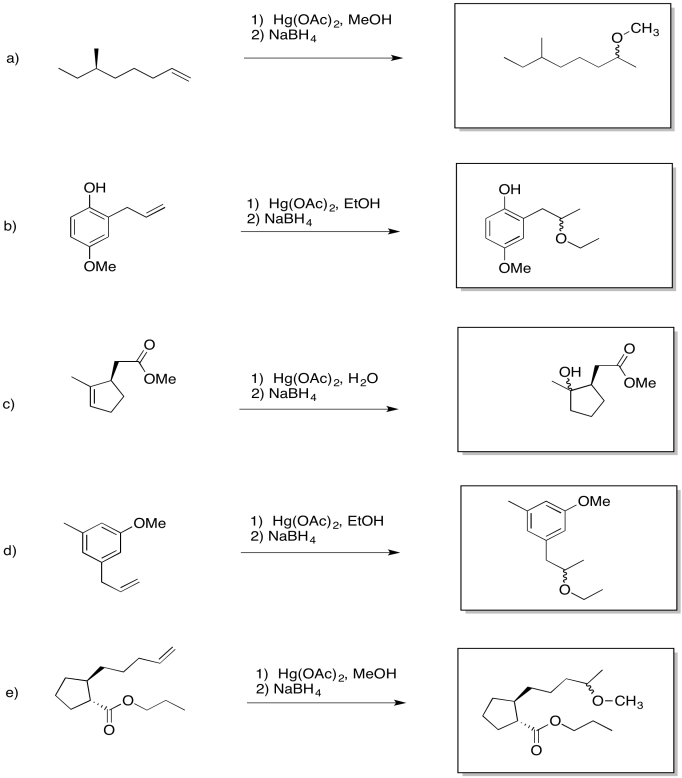
Problem EA6.1.
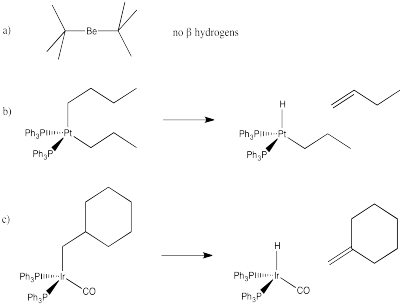
Problem EA6.2.
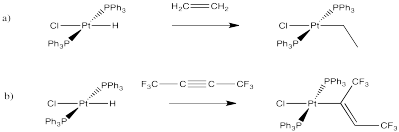
Problem EA6.3.
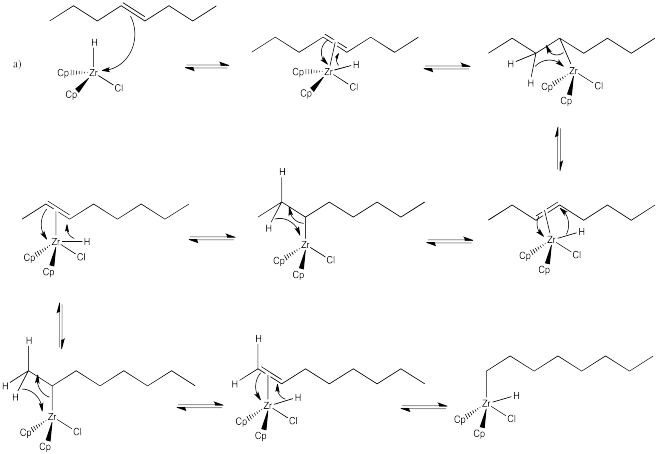
Problem EA6.4.
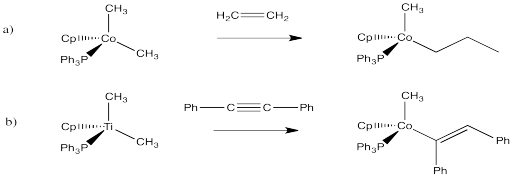
Problem EA6.5.
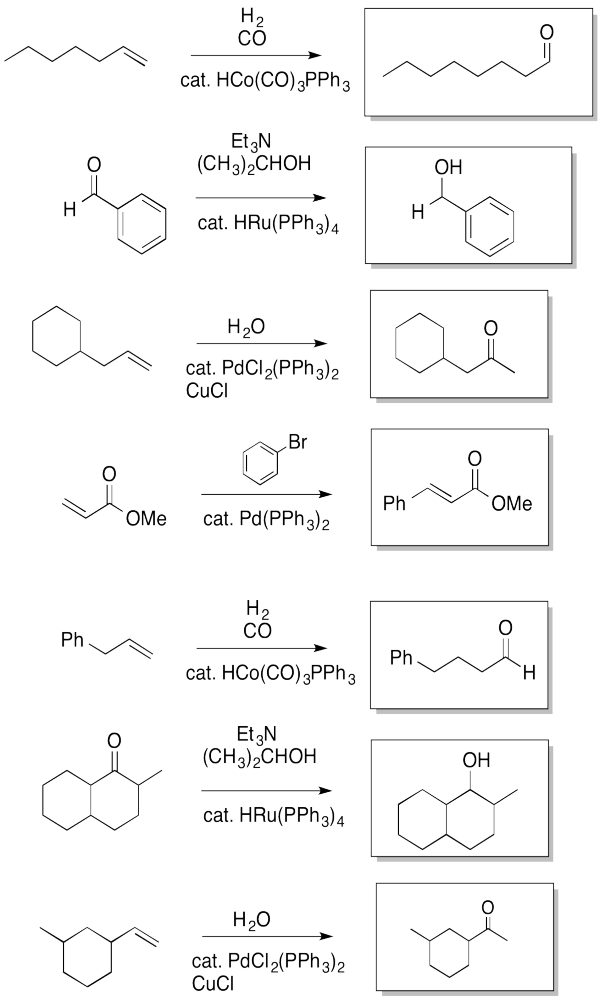
Problem EA6.6.
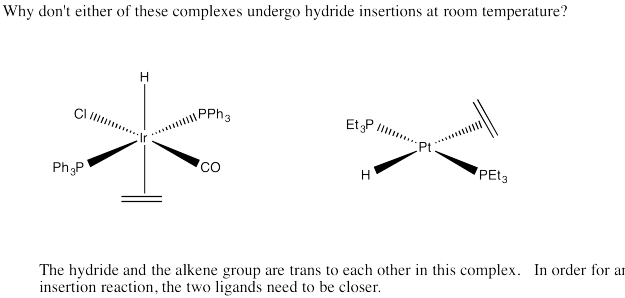
Problem EA6.7.
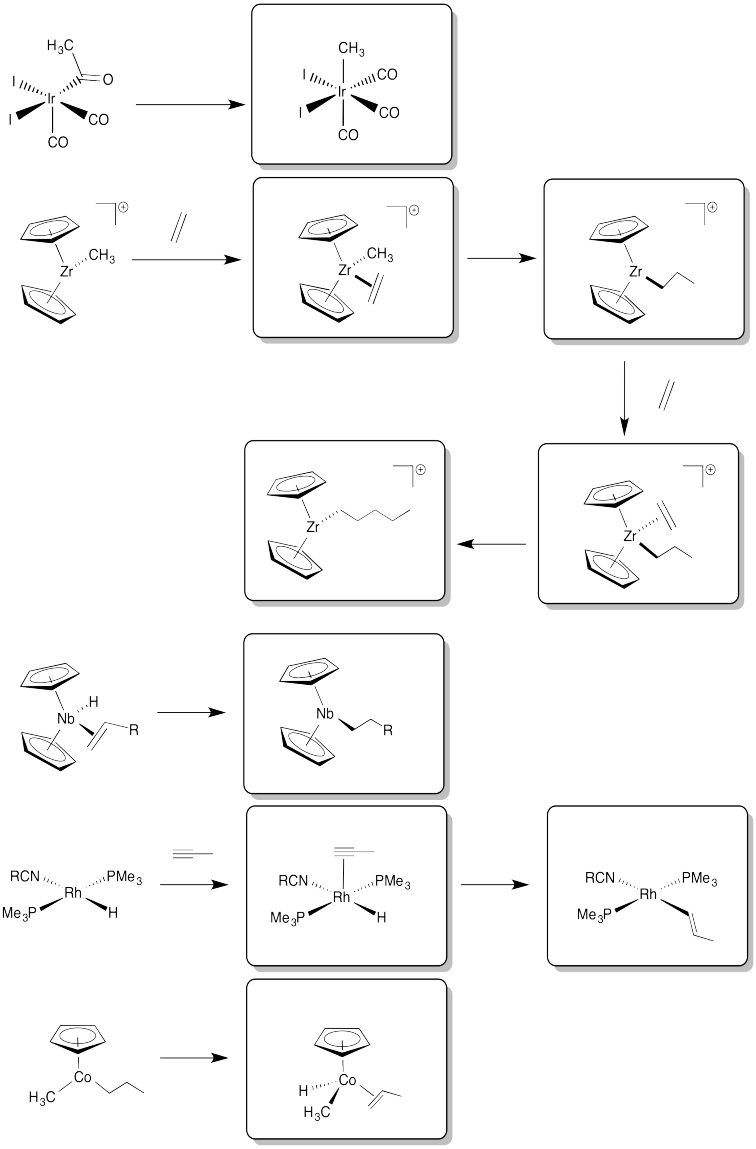
Problem EA6.8.
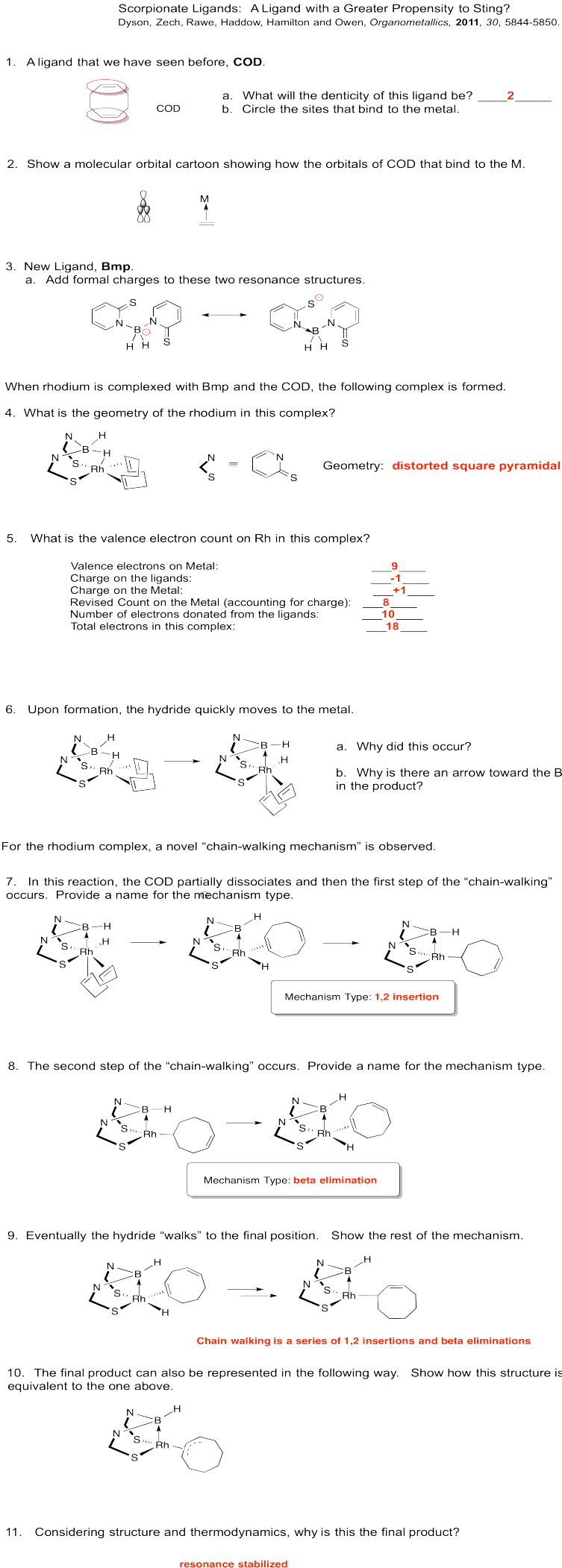
Problem EA6.9.
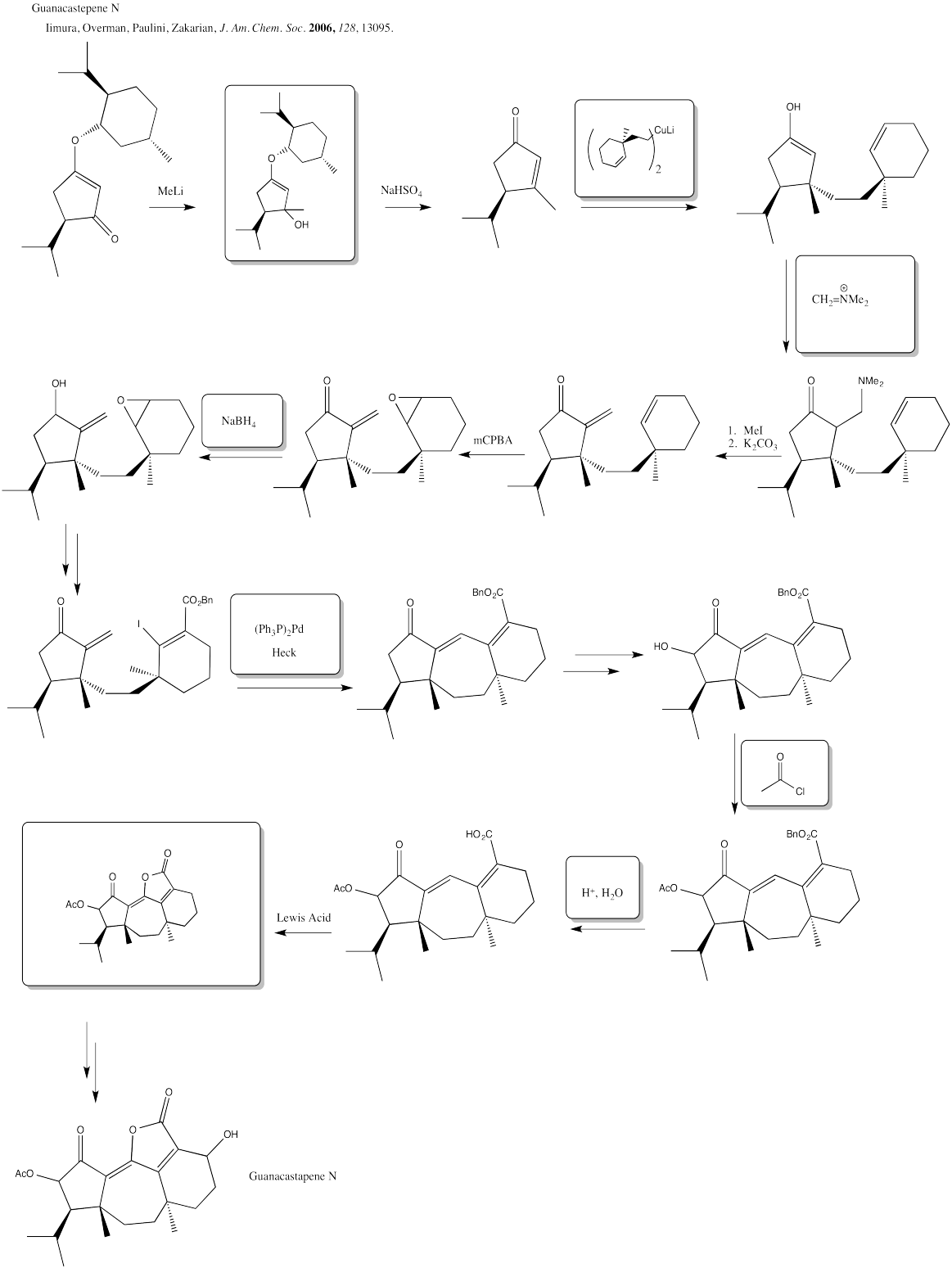
Problem EA6.10.
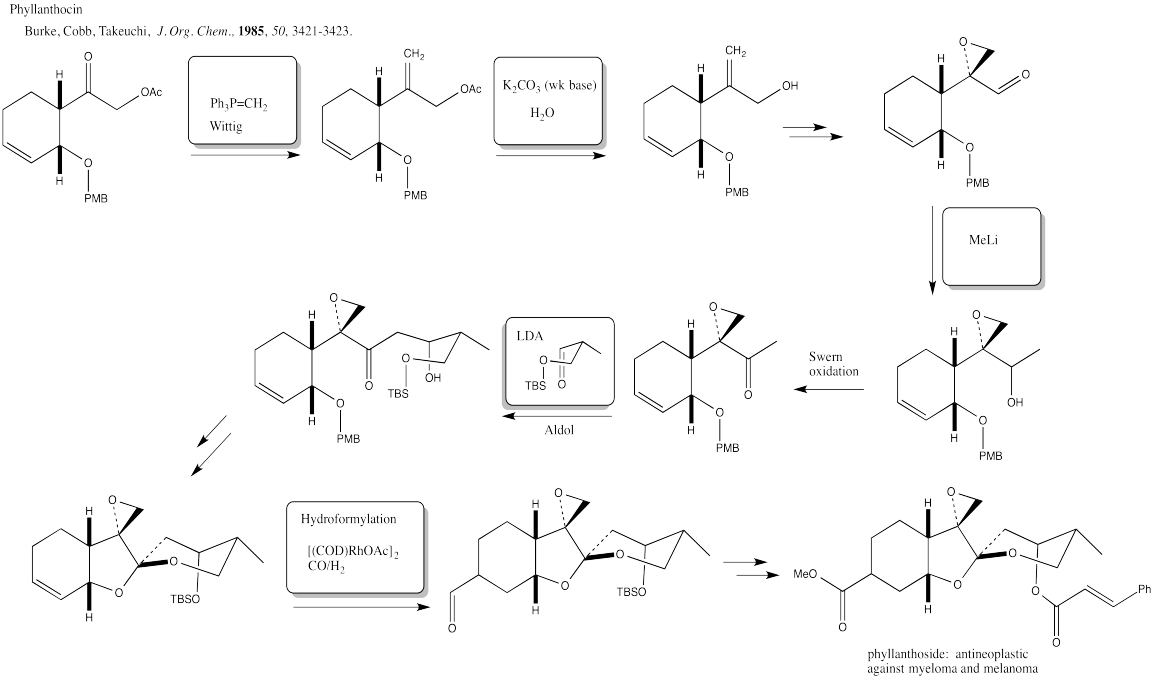
Problem EA7.2.
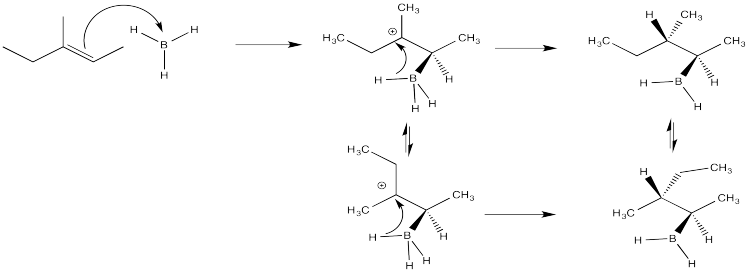
Problem EA7.3.
Crowding is more severe in the structure on the left than in the structure on the right. The structure on the right, representing an approach to the transition state of the reaction, is more favourable than the other one.

Problem EA7.4.
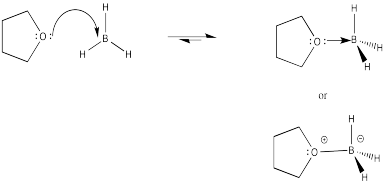
Problem EA7.5.
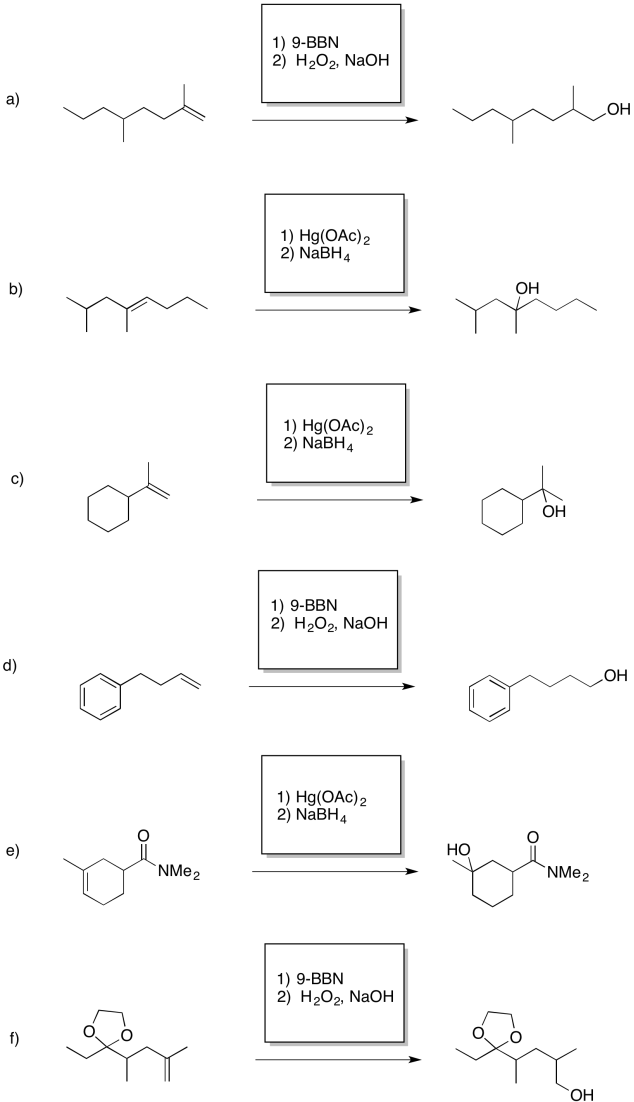
Problem EA7.6.
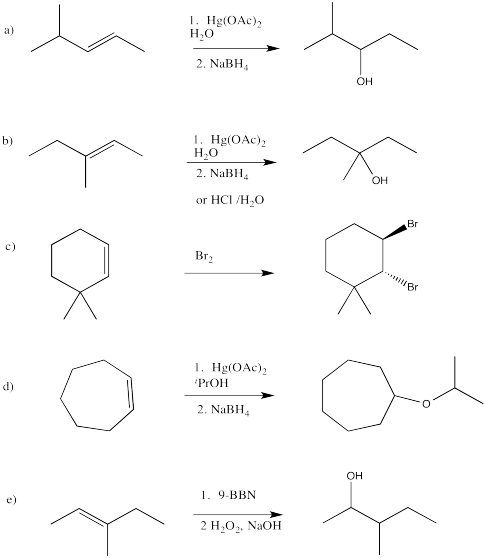
Problem EA7.7.
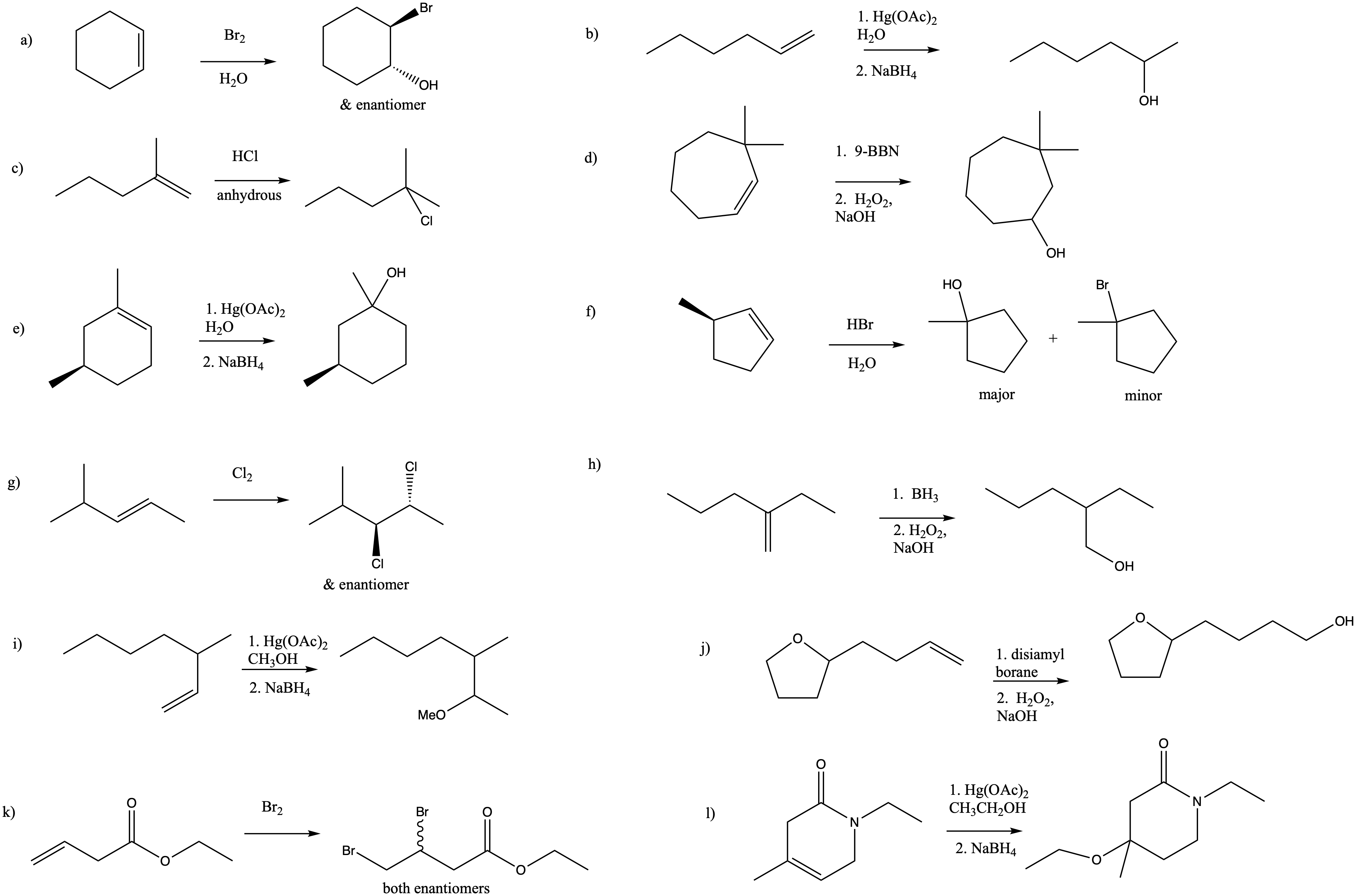
Problem EA8.1.
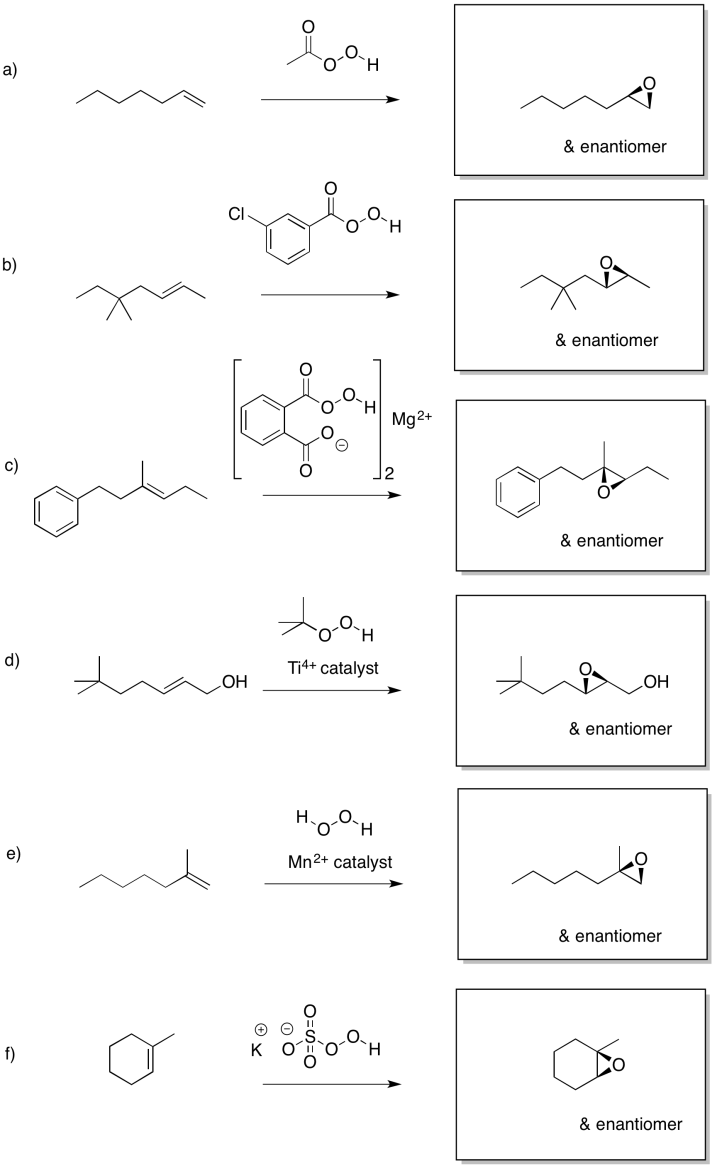
Problem EA8.2.
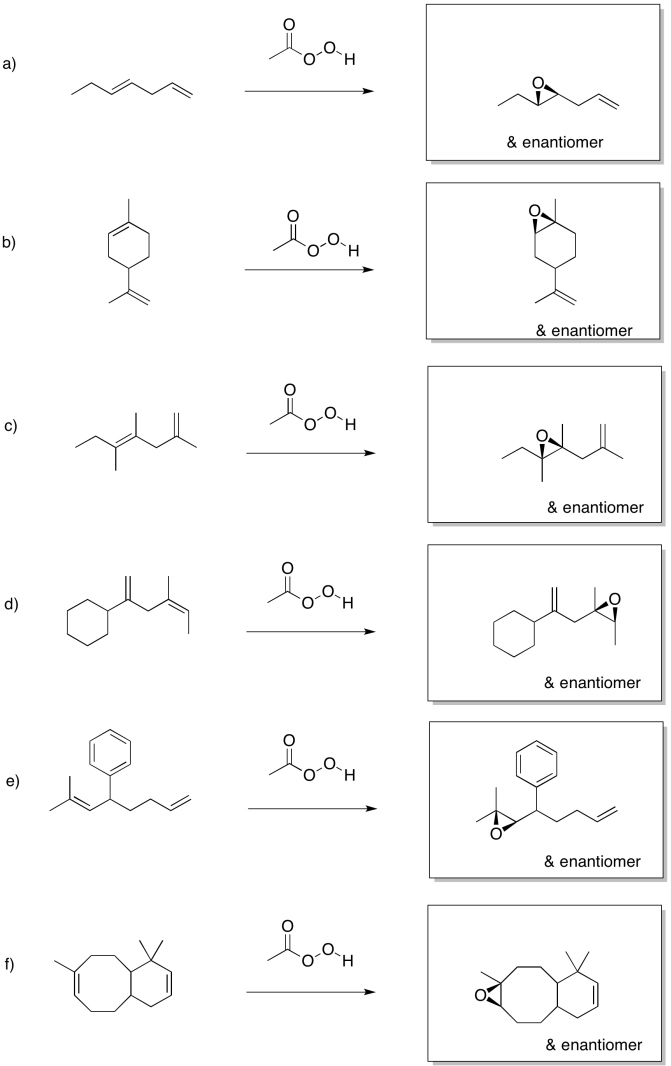
Problem EA8.3.
Problem EA8.4.
D-(-)-tartrate is the (2S,3S)-isomer. L-(+)-tartrate is the (2R,3R)-isomer. Each chiral center is configured opposite to the corresponding one in the other molecule, so the molecules are enantiomers.
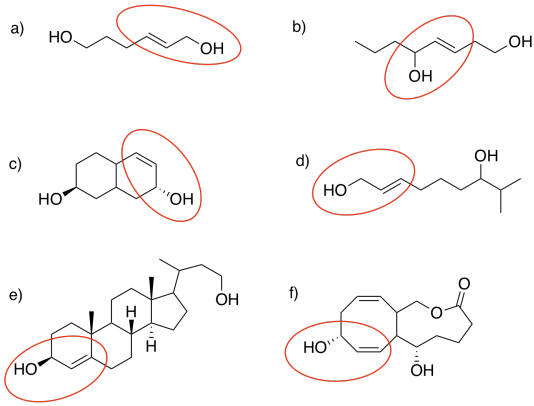
Problem EA8.5.
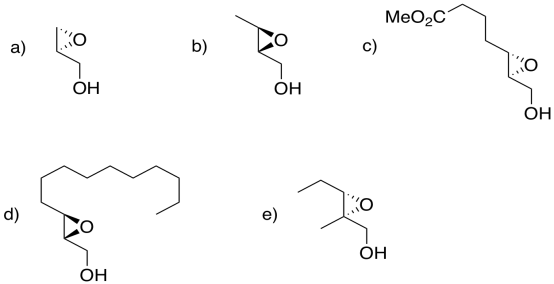
Problem EA8.6.
Problem EA8.7.
Problem EA8.8.
Problem EA9.1.
The chlorines can (weakly) share their electrons to fill the octet on carbon.
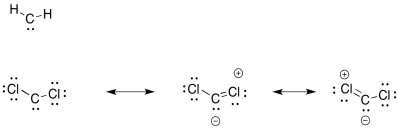
Problem EA9.2.
The oxygen can π-donate to help fill the octet on the carbon.
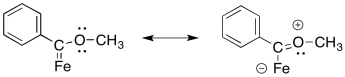
Problem EA9.3.
Not only can the nitrogens π-donate to help fill the octet on carbon, but this is an aromatic system. It is planar, cyclic, fully conjugated, with an odd number of electron pairs in the π-system.
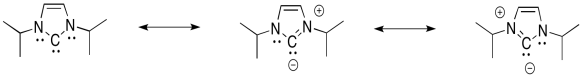
Problem EA9.4.
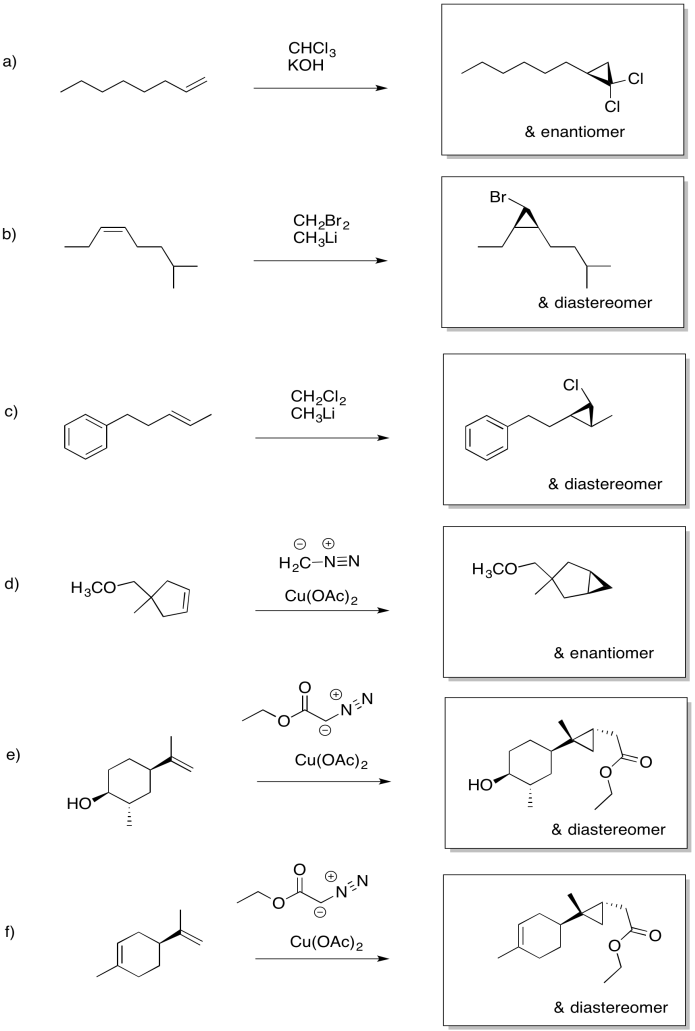
Problem EA10.1.
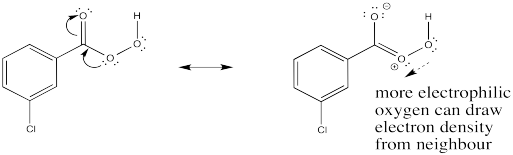
Problem EA10.2.
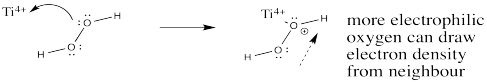
Problem EA10.3.
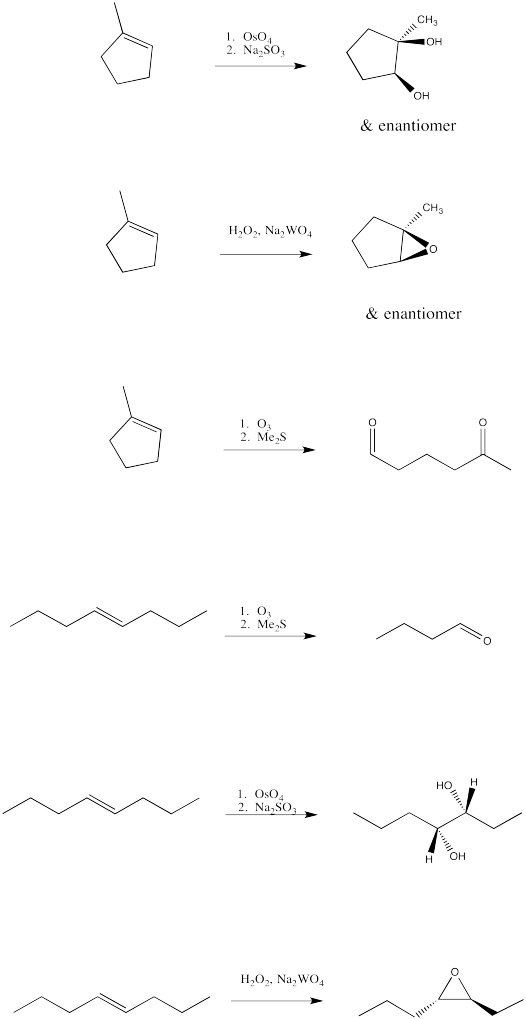
Problem EA10.4.
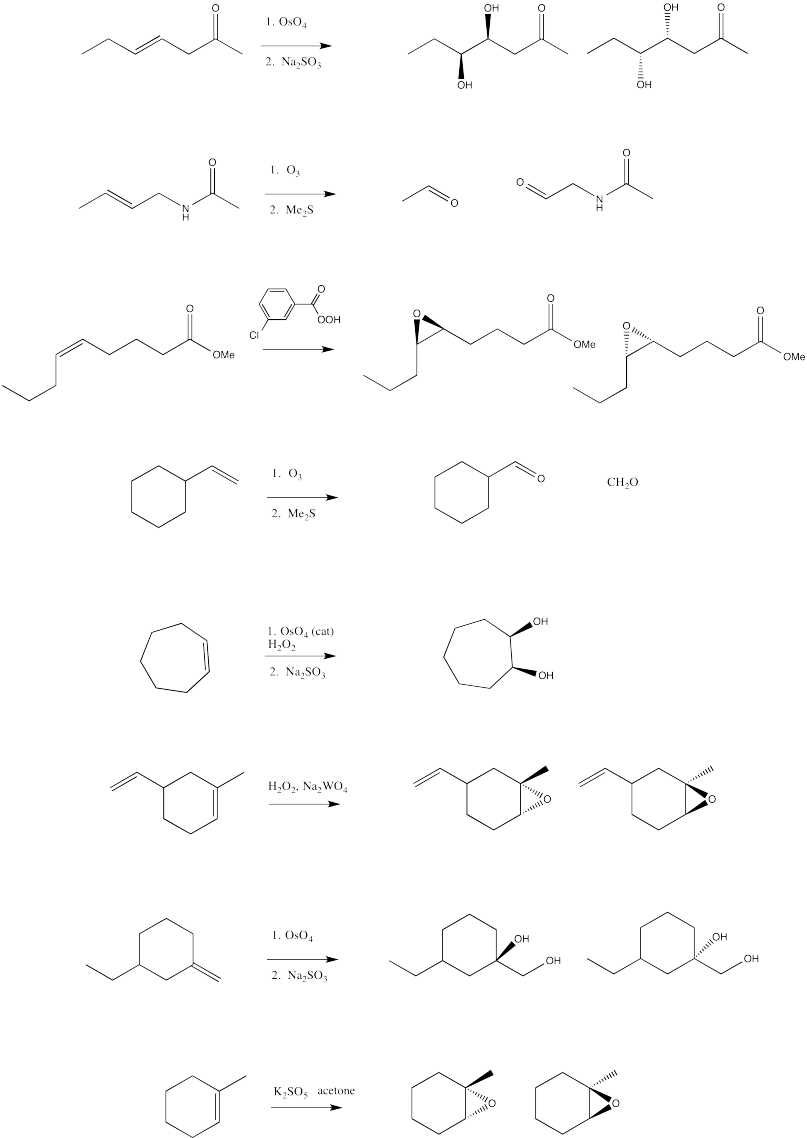
Problem EA10.5.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: