Reactivity in Chemistry
Enzyme Catalysis
EZ5. Types of Reversible Inhibitors
Reversible inhibitors are extremely important in regulating enzyme activity. Unlike irreversible inhibitors, they do no shut down an enzyme completely by permanently disabling it. They are much more subtle, just slowing it down temporarily. There are a number of different ways that the inhibitor could do that, however, and so we will take a look at those possibilities here.
The simplest idea is that an inhibitor can bind in the active site of the enzyme. If the inhibitor is already bound there, the substrate cannot. The inhibitor is in the way. This is called competitive inhibition.

Figure EZ5.1. Competitive inhibition.
This case is a true competition. If there are more inhibitor molecules than substrate molecules, the inhibitors will probably win out, blocking the substrate from entering the active site. But if there are more substrate molecules than inhibitor molecules, then chances are the substrate will be able to bind, and the subsequent reaction will proceed.
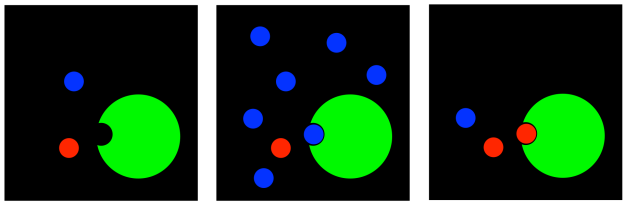
There are a number of cases, however, in which the inhibitor does not bind at the active site at all. It binds someplaces else on the enzyme, at a place called an allosteric site. When the inhibitor is bound at the allosteric site, it somehow interferes with the function of the enzyme. An inhibitor that binds at a site other than the active site is generally called an allosteric inhibitor.
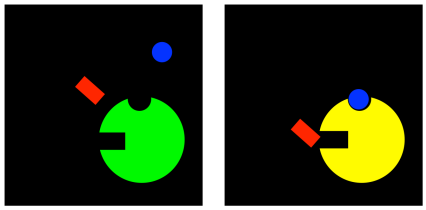
Figure EZ5.2. Allosteric inhibition.
Allosteric inhibitors can work in a few different ways. In perhaps the simplest case, when the allosteric inhibitor binds to the enzyme, it causes some sort of conformational change that prevents the enzyme from carrying out reactions. It doesn't interfere with substrate binding, so the substrate can still complex with the enzyme, but nothing will happen after that. This type of inhibition is called noncompetitive inhibition, or sometimes pure noncompetitive inhibition, for the simple reason that the inhibitor is not interfering directly with the substrate; it's temporarily disabling the enzyme in some other way.
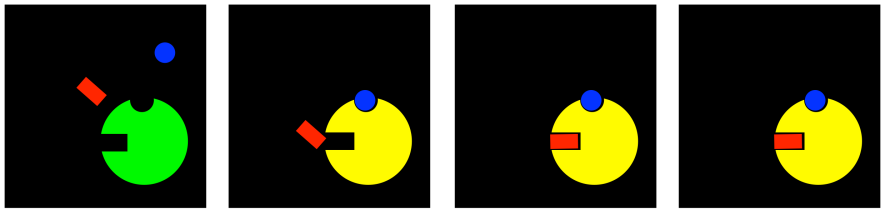
Figure EZ5.3. Noncompetitive inhibition.
Noncompetitive inhibition interferes with the machinery of the enzymatic reaction, but leaves substrate binding alone. But if allosteric inhibitors cause some sort of conformational change in the enzyme, then it's easy to imagine they could somehow mess up the binding site. As a result, they might interfere with substrate binding, even without being in direct competition with the substrate. This mode is called mixed noncompetitive inhibition. Although the inhibitor is not directly competing for the same binding site as the substrate, it ends up preventing the substrate from binding anyway.
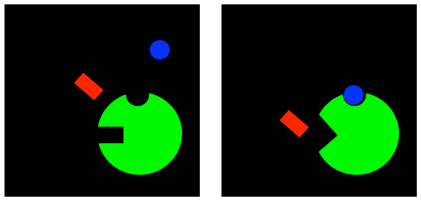
Figure EZ5.4. Mixed inhibition.
Well, that game can go both ways. If an inhibitor can change the binding site of the substrate, maybe the substrate can change the binding site of the inhibitor. In uncompetitive inhibition, the inhibitor is not able to bind to the free enzyme. However, when the substrate binds, it induces a conformational change in the allosteric site, allowing the inhibitor to bind. If the inhibitor binds, it interferes with the machinery of the enzyme, so the enzyme can't do its job, even though the substrate is bound.
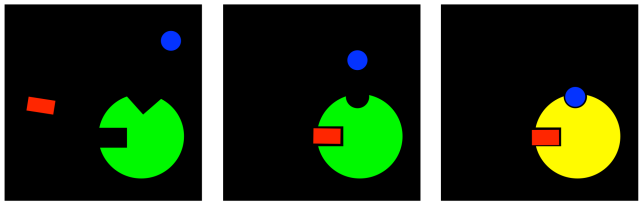
Figure EZ5.5. Uncompetitive inhibition.
There is one more case that is a complemetary idea; it isn't about inhibition at all. If a compound can bind at an allosteric site, changing the conformation of the active site so that a substrate can no longer bind, then maybe the reverse is true. Maybe a compound can bind at an allosteric site, allowing a substrate to bind that previously could not.
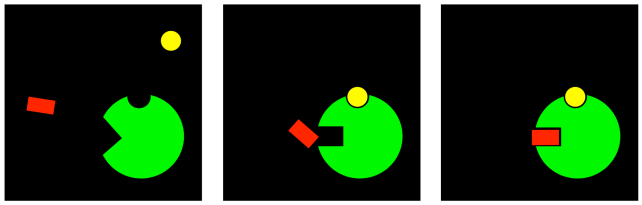
Figure EZ5.6. Allosteric activation.
Instead of inhibiting the enzyme, this compound would be activating it. It would make binding possible, and suddenly the enzyme could do ts job. This compound is an allosteric activator. As in inhibition, there may be different modes through which the allosteric activator could turn the enzyme on. The important thing is to know that in order to regulate an enzyme fully, there nood to be both on and off switches. Activators turn on enzymes that are waiting to be used. Inhibitors turn off enzymes that we don't need right now.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: