Reactivity in Chemistry
Fatty Acid Synthesis
FA4. Solutions to Selected Problems
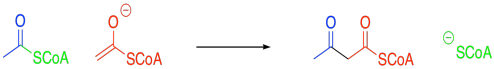
Problem FA2.1.

Problem FA2.2.
The product becomes conjugated. In general, the more conjugation there is in the product of an aldol addition, the more likely is a subsequent condensation (elimination or dehydration). However, other conditions can lead to the loss of water.
Problem FA2.3.
Entropy. The dehydration or elimination takes one molecule (the beta-hydroxy thioester) and converts it into two molecules (the water and the alpha,beta-unsaturated thioester. That change represents an increase in internal entropy. Because the entropy term in free energy is weighted by temperature (ΔG = ΔH - TΔS), it predominates as the temperature rises.
Problem FA3.1.
In the case of the malonyl enolate, there is additional delocalisation as demonstrated by resonance. This anion has extra stability.
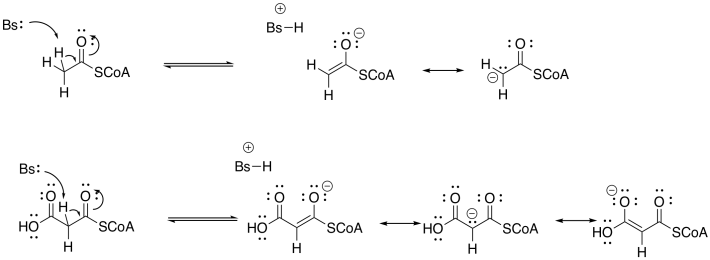
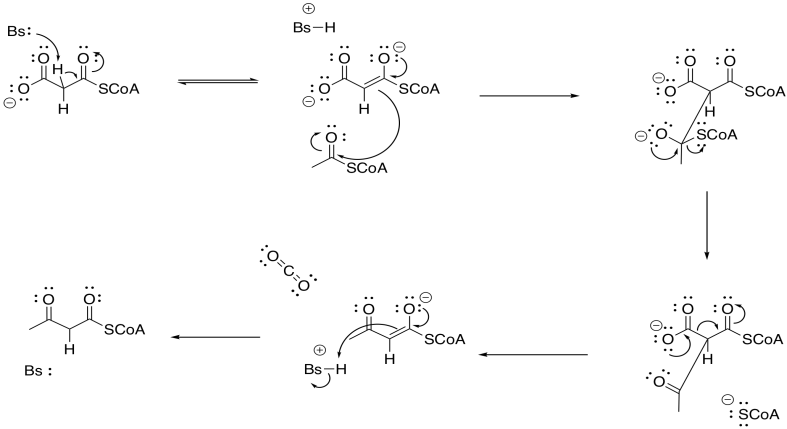
Problem FA3.2.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: