Reactivity in Chemistry
Understanding Mechanism
UM1. Intermediates
By now you are familiar with a range of reaction types in organic, inorganic, and biochemistry. The purpose of this chapter is to help you review some of the tools that we use in communicating how reactions happen.
First and foremeost, a mechanism is a sequence of intermediates. What happens to the structure of the compound as it undergoes chemical change? Does that change happen all at once, or does it happen in stages? If it happens in stages, what kinds of intermediates are involved?
Let's review some different kinds of reactive intermediates that may occur along a reaction pathway. These intermediates are not particularly stable, and so they go on to react further until they form more stable products.
Cations
Cations and anions can be unstable for the simple reason that charge separation costs energy. There are a few cases in which these ions are really quite stable -- alkali cations such as Na+ and halide anions such as Cl- come to mind -- but here we are interested in exploring the less stable, more temporary examples of ions.
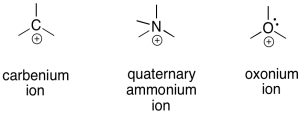
Unlike sodium ions, cations of carbon, nitrogen, or oxygen are reactive. These relatively electronegative atoms are not very stable with a positive charge.

Figure UM1.1. Some reactive intermediates.
Carbocations, or carbenium ions, in which the positive charge is on a carbon atom, are generally unstable. Carbon is in the upper right part of the periodic table, so it is not particularly electropositive like sodium. A positive charge on carbon frequently makes a molecule reactive. Nevertheless, this intermediate is frequently encountered during organic reactions.
Are all carbocations equally unstable? No. In general, there are two main factors that stabilize carbocations. The first, and most important, is the degree of substitution. A tertiary carbocation, in which the carbon with the positive charge is attached to three other carbon atoms, is fairly stable. A primary carbocation, in which the carbon bearing the positive charge is attached to only one other carbon and two hydrogen atoms, is not so stable. A secondary carbocation, with the positive carbon attached to two other carbons and a hydrogen atom, is intermediate in stability. Of course, a methyl cation, in which a positive carbon is attached to three hydrogen atoms, is not very stable at all.
The reasons for these differences are sometimes explained in terms of hyperconjugation. According to this idea, weak interactions between the unoccupied p orbital on the positive carbon and the occupied sigma bonds on the neighbouring carbons can stabilize the cation somewhat. Very loosely, imagine these bonds, which are made of pairs of electrons, can allow a little bit of negative charge to overlap with the cation, lowering its overall positive charge just a tad. More correctly, the empty p orbital can interact with the sigma bonds to produce two molecular orbital combinations; one of these is an in-phase combination and is lower in energy than either of the original orbitals, whereas the other, out-of-phase combination is a little higher in energy. Because only two electrons are involved, from the sigma bond, both can get to a lower energy level this way. They both drop into the lower energy combination. In that sense, the cation is stable not just because the positive charge is any less but because the neighbouring bonds can drop lower in energy.
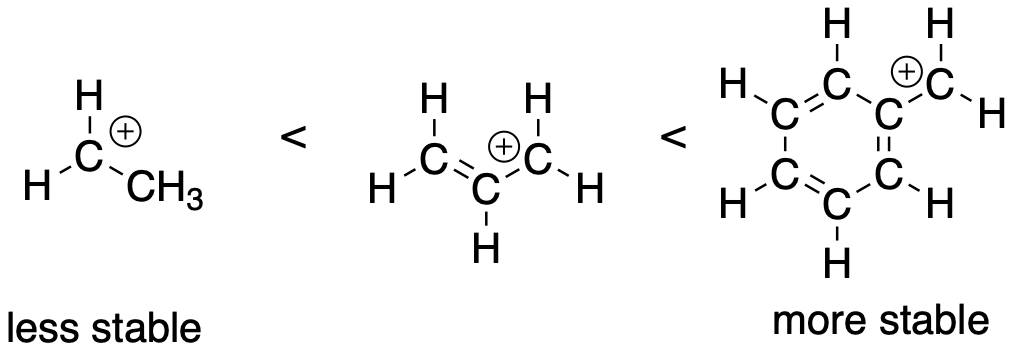
Figure UM1.2. Increasing degree of substitution as a stabilizing factor in carbocations.
The second factor that stabilizes positive charge is resonance delocalization. If a double bond is adjacent to a cation, conjugation between filled and empty p orbitals allows the porisitve charge to be deistributed across multiple carbon atoms. This effect lowers the amount of positive charge borne by an one carbon atom. this kind of delocalizing effect is very common in stabilizing reactive intermediates. For this reason, allylic (CH2=CH-CH2+) and benzylic cations (C6H5CH2+) are particularly stable. They are about as stable as a secondary cation along a regular carbon chain, even if they would otherwise be only primary cations.
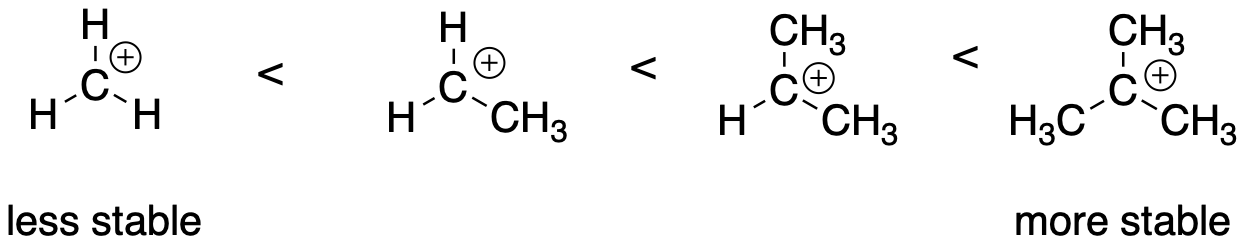
Figure UM1.3. Resonance as a stabilizing factor in carbocations.
Of course, other atoms can be cations, too. As seen above, oxygens and nitrogens are very commonly encountered as cations. That is partly because they are very good at donating electrons to neighbouring atoms in need.
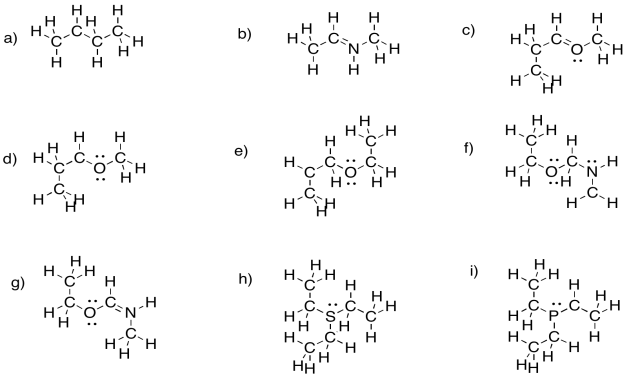
Problem UM1.1.
Identify the positive atom in each of the following molecules.
Problem UM1.2.
Within each group, rank the cations from most stable to least stable.
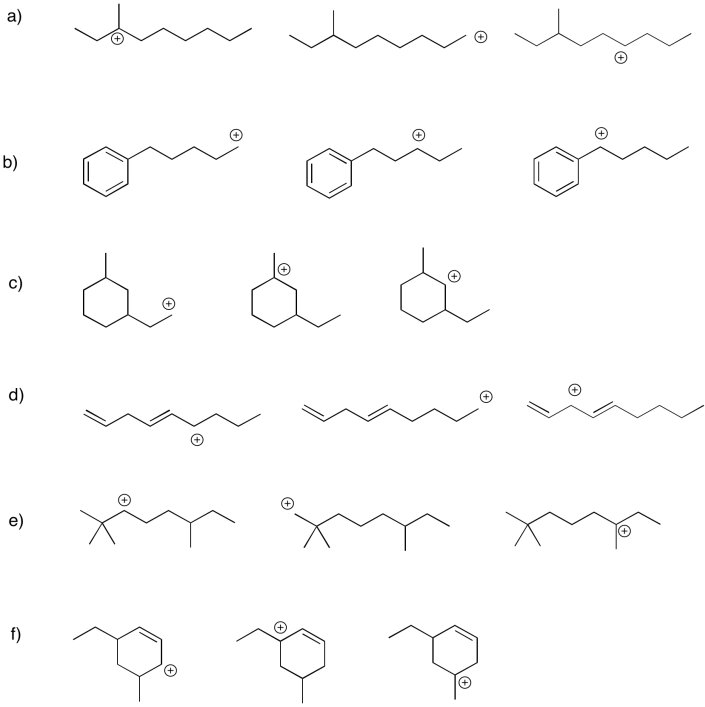
Problem UM1.3.
Sometimes, remote groups provide additional stabilization for a cation. Indicate whether each of the following cations would be more stable or less stable than a benzyl cation, and explain why.
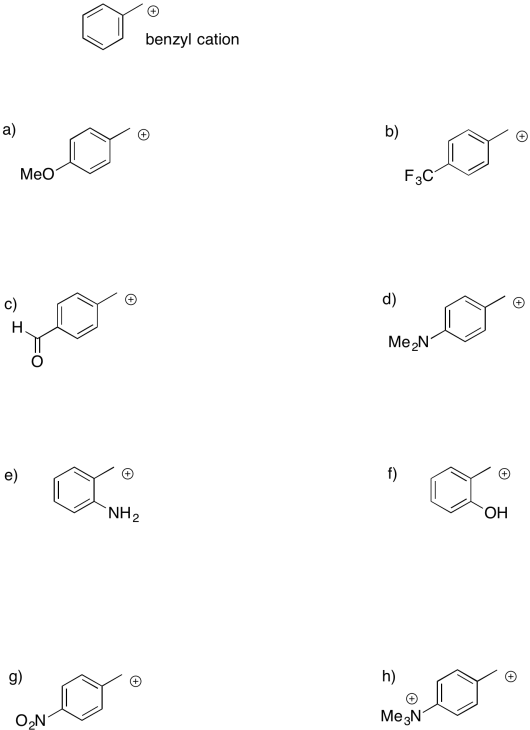
There are other, more subtle factors that can influence the stability of cations. Charge stability is affected by the structure further away from the atom bearing the charge. For example, a triethylammonium cation and a trimethylammonium cation look pretty similar. However, a triethlammonium cation is a little less stable than a trimethylammonium cation, at least in solution.
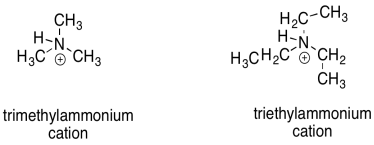
Figure UM1.4. Some alkylammonium cations.
The difference in these cations is related to the size of the overall molecule. Reactions usually take place in a solvent. The solvent plays an important role; it allows the reactants to move around, moderates heat flow, and may even provide lone pairs or protons to aid in acid/base reactions. A cation or anion most commonly occurs in solution. Because charge stability is a big issue, the solvent will also help to stabilize the charge. To do so, the solvent molecules will arrange themselves in a favourable way around the cation. The bigger the cation, the more solvent molecules will be needed to arrange themelves around it.
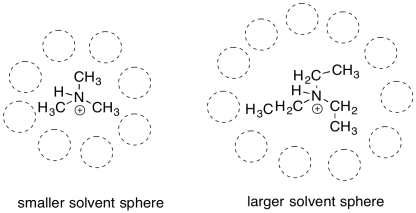
Figure UM1.5. Solvent spheres around alkylammonium cations.
It's worth noting that not all of these ionic species are necessarily intermediates. Alkylammonium ions, for example, are stable enough to be prepared as salts and stored in a bottle. Carbocations are not really stable enough to do that. Part of the difference is that carbocations have a low-lying empty p orbital. Although alkylammonium cations are also positiviely charged, they are much less electrophilic, partly because they don't have such an accessible, low-energy acceptor orbital.
Anions
Negatively charged ions are also common intermediates in reactions. Like cations, anions are frequently unstable species. These species are stabilized by a number of different factors, not unlike cation stability.
Carbanions, amide ions and alkoxide ions are examples of anionic intermediates.
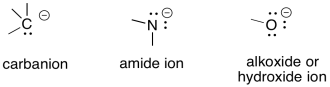
Figure UM1.6. Some anionic species.
Remember, there are just a few key factors that explain a great deal of questions about anion stability.
Within a column of the periodic table, when comparing two atoms with negative charge, the stability of the anions principally depends on polarizability of the atom. Polarizability refers to how easily distorted the electrons are around the atom. The larger the atom, and the further the electrons from the nucleus, the more polarizable it is. The more polarizable the atom, the more stable the anion.
Within a row of the periodic table, the more electronegative an atom, the more stable the anion.
Problem UM1.4.
Within each group, rank the anions from most stable to least stable.
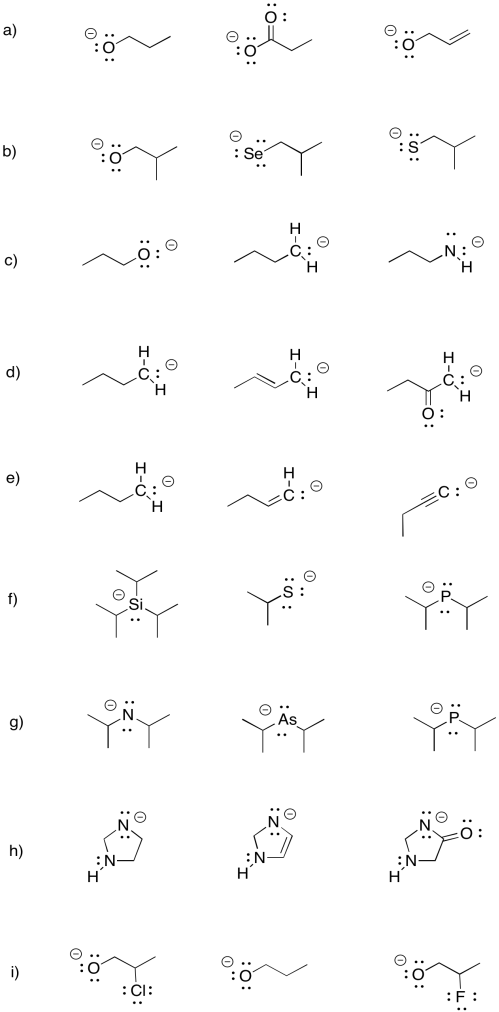
Problem UM 1.5.
Sometimes, remote groups provide additional stabilization for a cation. Indicate whether each of the following anions would be more stable or less stable than a phenoxide anion, and explain why.
Radicals
Radicals are species with an unpaired electron. They are reactive because they are short an octet, but the presence of an unpaired electron means they react in a different way from typical electrophiles. Carbon, nitrogen, and oxygen compounds show some typical examples of radical structures.
![]()
Figure UM1.6. Some radical species.
Note that these radicals do not necessarily have charges. That is because they are bonding to one atom fewer than normal, but they are retaining just one of the electrons from the missing bond. In fact, radicals are often formed by breaking a bond within a normal, "closed-shell" compound, such that each atom involved in the bond takes one of the electrons with it. This is called "bond homolysis" and implies the bond is split evenly between the atoms. In contrast, "bond heterolysis" means the bond is broken unevenly, with one atom taing both of the electrons.
Problem UM1.6.
Confirm that there is no formal charge in each of the species shown above.
Radical ions are also possible. Radical anions can result from the addition of an extra electron to a normal, closed-shell compound. Radical cations can result through the removal of an electron from a normal, closed-shell compound.
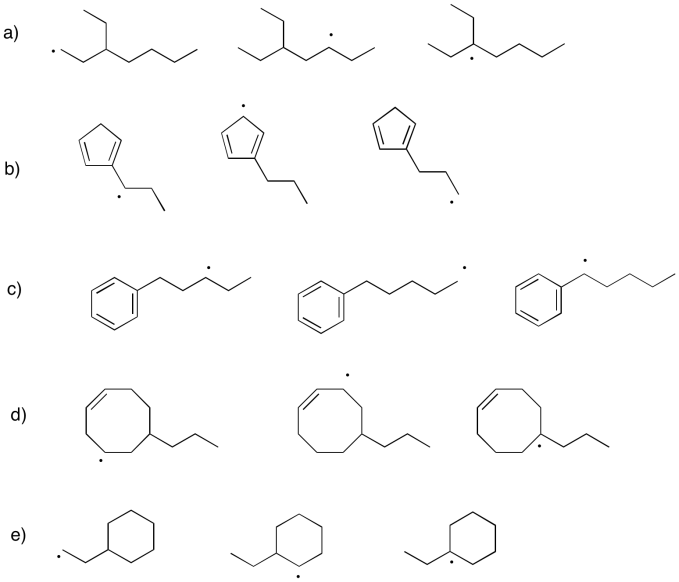
Because radicals are electron-deficient species, in the sense that they lack an octet, they are often stabilized by the same factors that would stabilize a cation. In particular, they are stabilized by resonance delocalization, and carbon radicals are more stable on more-substituted carbons than on less-substituted carbons, just like cations. However, they are generally less sensitive that cations to these factors, because they do not actually have a positive charge.
Problem UM1.7.
Within each group, rank the radicals from most stable to least stable.
Carbenes and Nitrenes
We don't often see carbenes and the related nitrenes, but they are important intermediates in synthetic processes involving electrophilic addition to alkenes. Carbenes and nitrenes are two electrons short of an octet, but do not have a formal charge.
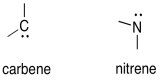
Figure UM1.7. The general structure of a carbene and a nitrene.
Carbenes are unusual because they can be thought of as both electrophiles or nucleophiles. The have lone pairs -- the usual requirement for a nucleophile. They also have an empty orbital, which would typically make them electrophiles.

Figure UM1.8. The dual nature of a carbon, in which the same carbon atom is both an electron-donor and an electron-acceptor.
Because they lack an octet, carbenes and nitrenes can be stabilized through pi-donatin.
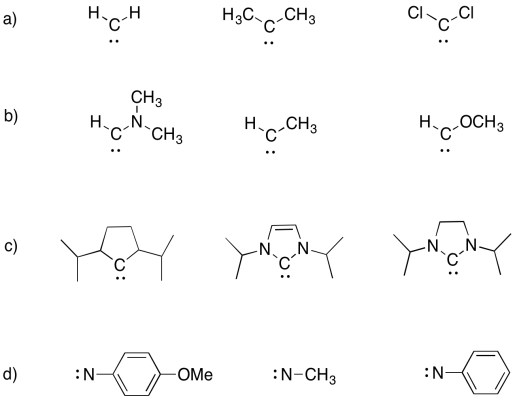
Problem UM1.8.
Arrange the following carbenes in order from most stable to least stable.
Coordination Complexes
Coordination complexes are not necessarily reactive intermediates, but they often use species as ligands that would be reactive intermediates on their own.
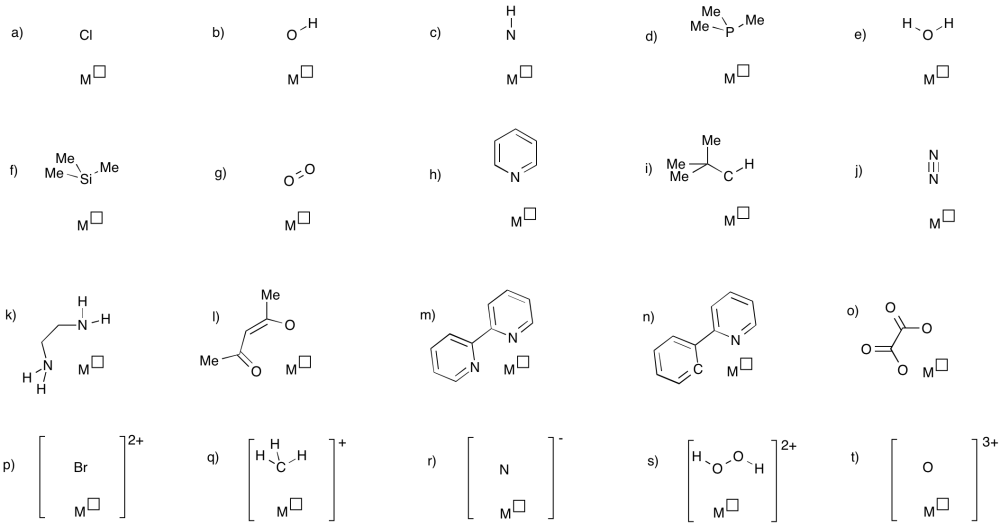
Problem UM1.8.
In the following pictures, decide whether the ligand is an anionic or neutral donor. Use the correct symbol (a line or an arrow) to stand for the ligand-metal bond. Assign the oxidation state to the metal to satisfy the overall charge.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.