Reactivity in Chemistry
Understanding Mechanism
UM3. Solutions to Selected Problems
Problem UM1.1.

Problem UM1.2.
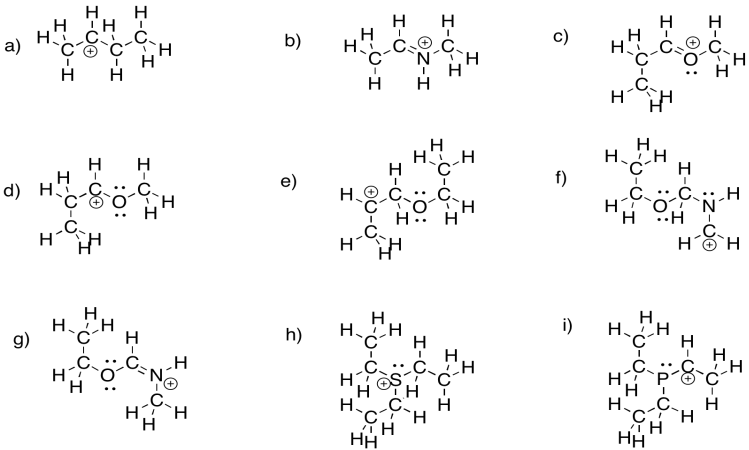
Problem UM1.3.
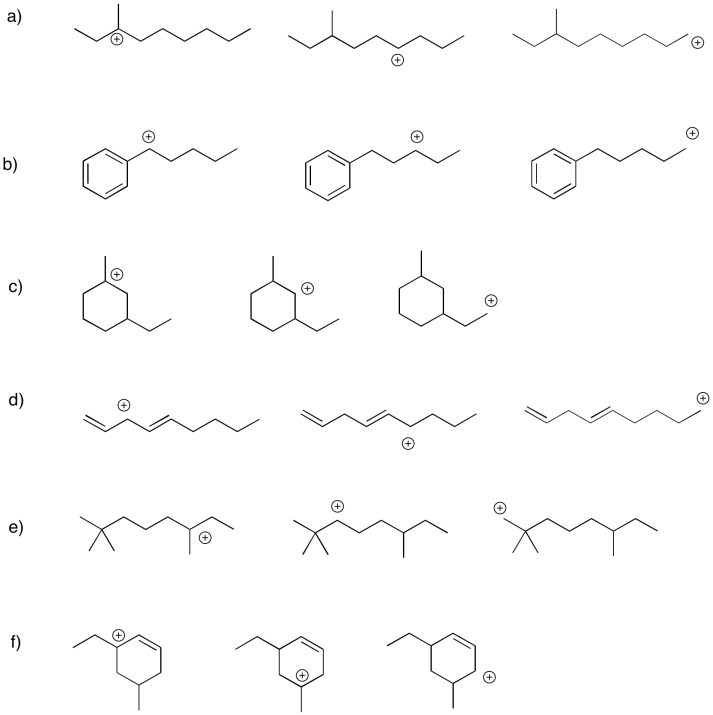
Problem UM1.4.
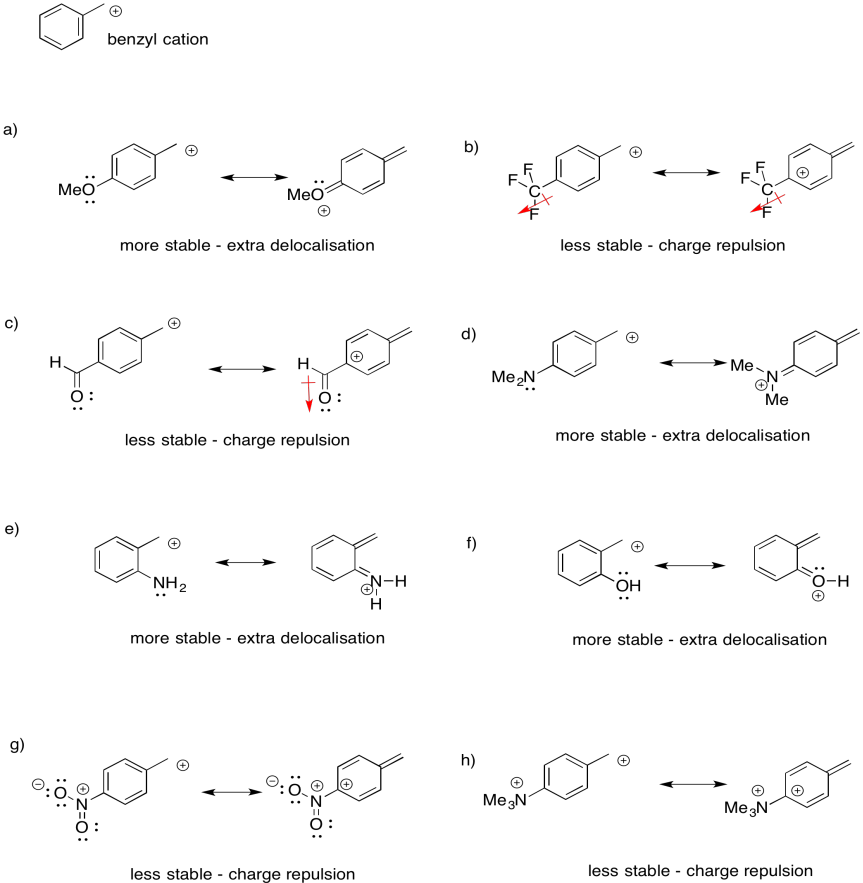
Problem UM1.5.
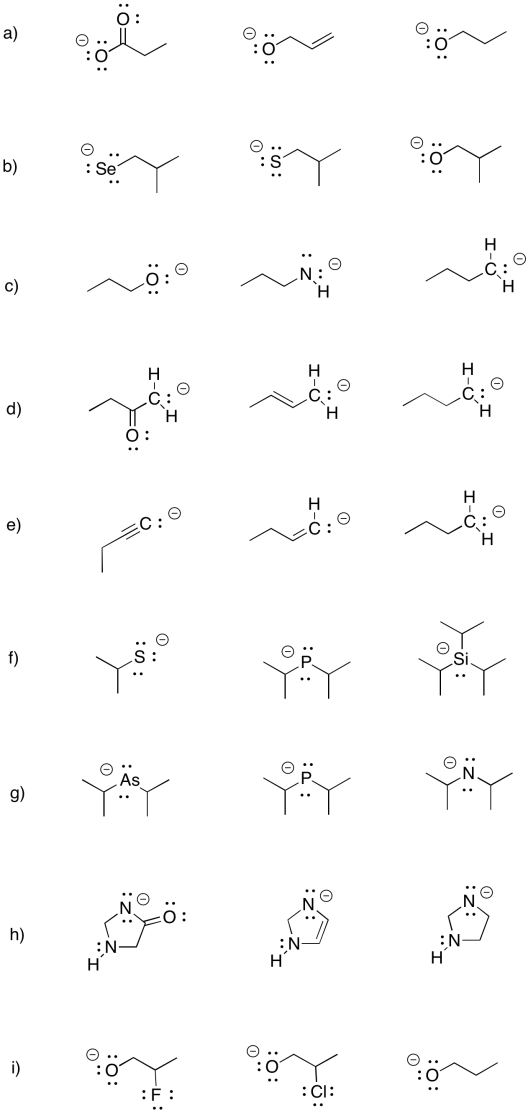
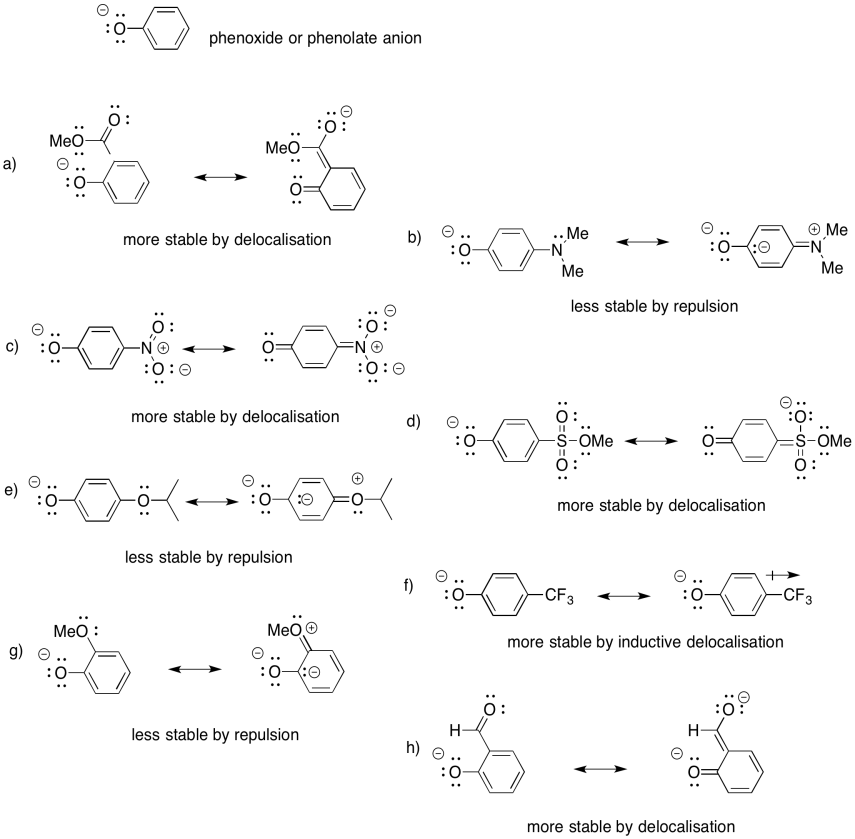
Problem UM1.6.
a) formal charge = # e- in periodic table - # e- nonbonding - (# e- in bonds)/2
formal charge = 4 - 1 - 6/2 = 0
b) formal charge = # e- in periodic table - # e- nonbonding - (# e- in bonds)/2
formal charge = 5 - 3 - 4/2 = 0
c) formal charge = # e- in periodic table - # e- nonbonding - (# e- in bonds)/2
formal charge = 6 - 5 - 2/2 = 0
Problem UM1.7.
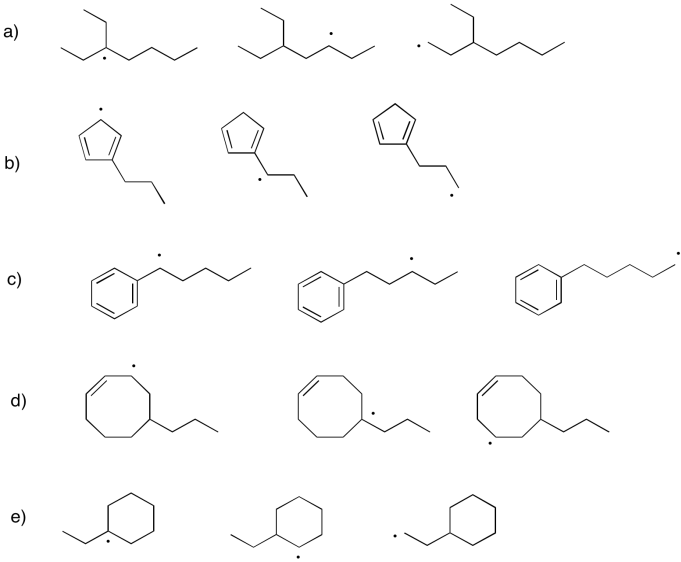
Problem UM1.8.
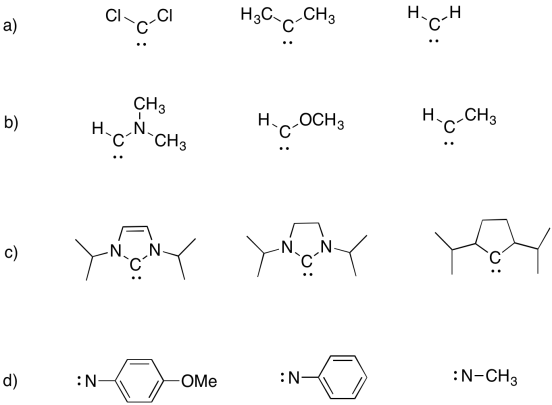
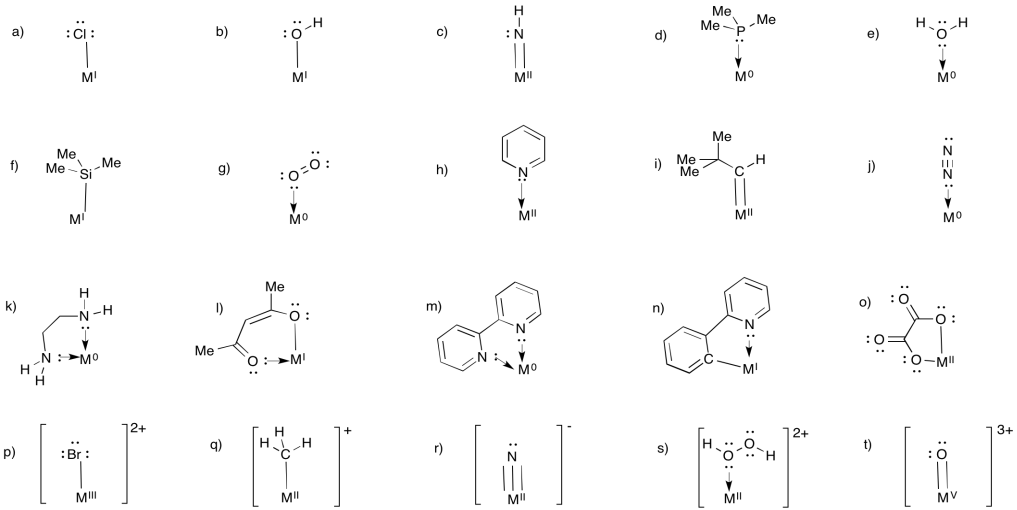
Problem UM1.9.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.