Oxidative Addition & Reductive Elimination
OA5. Oxidative Addition in Action: Catalytic Hydrogenation
Catalytic hydrogenation is a tremendously important reaction. It is an essential step in the synthesis of many fine chemicals as well as bulk commodities. In catalytic hydrogenation, a pair of hydrogen atoms are added across a double bond, turning an alkene into an alkane.
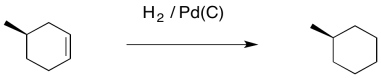
Figure OA5.1. A hydrogenation reaction.
In general, the reaction requires a large excess of hydrogen gas, often under high pressure. The reaction can be performed under either homogeneous or heterogeneous conditions. Homogeneous reactions employ a soluble catalyst. Soluble catalysts are those that dissolve under the reaction conditions; they often provide superior control over the reaction. Heterogeneous catalysts do not dissolve; they are solids that sit on the bottom of the reaction, like sand in a lakebed. One of the advantages of heterogeneous catalysts is that they can esily be filtered away from the rest of the reaction, making purifiction of the product much more straightforward.
Problem OA5.1.
Indicate whether the following mixtures are homogeneous or heterogeneous.
a) Kool-aid b) a glass of pop with ice c) orange juice d) cranberry juice
Because of the importance of hydrogenation, a number of catalysts have been developed over the years that are capable of performing the reaction. There are a number of motivations for working on catalyst development. One reason is speed: the faster the catalyst, the more product can be made and the more economical the process. Another reason is selectivity. Suppose there are two double bonds present in a molecule. Maybe you only want to hydrogenate one of these double bonds. By choosing the proper catalyst, you may be able to do that.
Let's take a look at a few different examples of catalysts with different selectivities.
Perhaps the most commonly used catalyst is palladium metal, a heterogeneous catalyst. Very often, expensive metals like palladium are not used in their pure state. For example, palladium is often dispersed on a "solid support", such as carbon. There are a couple of benefits of doing that. First, the expensive palladium metal is stretched a little further by mixing it with carbon, which is much cheaper. Usually, this mixture is about 5% palladium and 95% carbon, although different compositions can be used. In addition, use of a solid support helps to spread the metal particles out spatially. When the metal isn't all clumped together, it has an increased surface area. That means there are more places available for hydrogen and alkenes to bind and undergo the hydrogenation reaction. Finally, a solid support often tunes the reactivity of the metal that is stuck to it. The solid support might change the rate of the reaction or alter the selectivity because of interactions between the metal and the support.
Palladium on carbon, or Pd/C, provides an example of what we mean by selectivity. It is very good at adding hydrogen to alkenes. It can hydrogenate alkynes, too. However, it is not very good at hydrogenating more stable double bonds, such as those in conjugated dienes, or in benzene or other aromatics. In contrast, platinum oxide is much more general, hydrogenating regular alkenes and also conjugated ones.
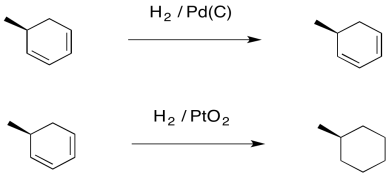
Figure OA5.2. Platinum oxide catalyzing a hydrogenation reaction that palladium on carbon cannot.
Under the right conditions, platinum oxide can even be used to hydrogenate benzene. That usually means very high pressure of hydrogen gas, equivalent to several hundred atmospheres of pressure.
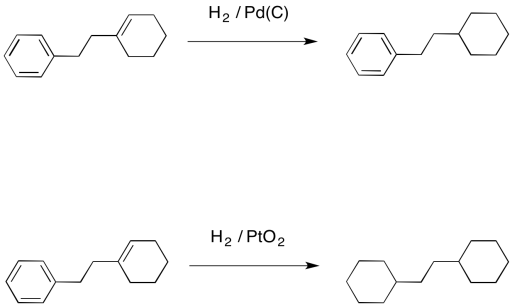
Figure OA5.3. Platinum oxide hydrogenating benzene.
Also, hydrogenation with palladium doesn't work very well with carbonyls. It usually won't reduce aldehydes or ketones. You may remember that other reducing agents (compounds that add hydrogen to carbon atoms) such as LiAlH4 can react with carbonyls quite easily, so palladium with hydrogen is very complementary to those reagents. Even PtO2 can't induce hydrogen to add across a carbonyl, although another heterogeneous catalyst, a ferocious one called Raney Nickel, can do the job.
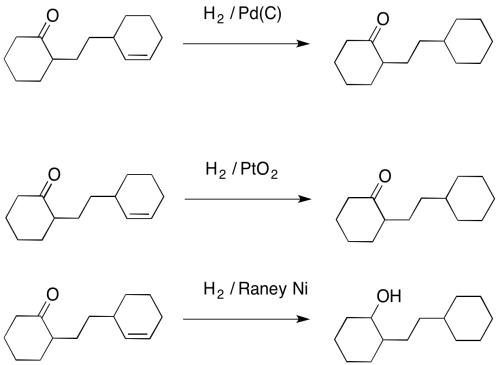
Figure OA5.4. Raney nickel hydrogenates alkenes, arenes and carbonyls.
However, not even Raney Nickel does very well at hydrogenating more stable carbonyls, such as amides, esters and carboxylic acids. Those are the ones at the bottom of the energetic "ski hill", so they are the least reactive carbonyls. They are difficult to hydrogenate, and are usually left alone.
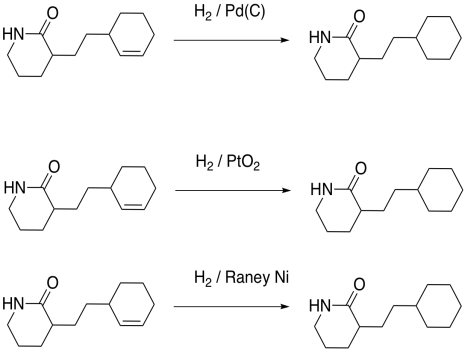
Figure OA5.5. Amides are resistant to hydrogenation.
On the other hand, palladium does just fine with some seemingly related compounds, containing imines and nitro groups. Although these groups contain multiple bonds and nitrogen, they do not have the same stability of amides. Imines and nitro groups behave a little more like simple carbonyl compounds when it comes to hydrogenation.
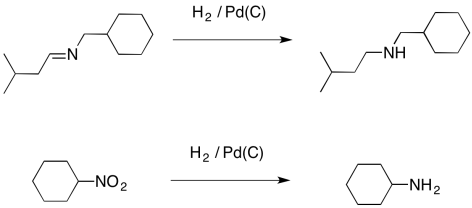
Figure OA5.6. Hydrogenation of imines and nitro groups.
All of these catalysts are examples of what are called heterogeneous catalysts. They are insoluble materials, including metals, metal oxides, and other solids. The reaction occurs on the surface of these solids, where the substrates are adsorbed along with hydrogen atoms from the hydrogen gas. The catalyst works using the principal of approximation, bringing reagents together in a specific location. It also brings specific reaction mechanisms for making and breaking the bonds necessary to carry out the reaction.
Figure OA5.7. An alkene and some hydrogen atoms bound to the catalyst surface..
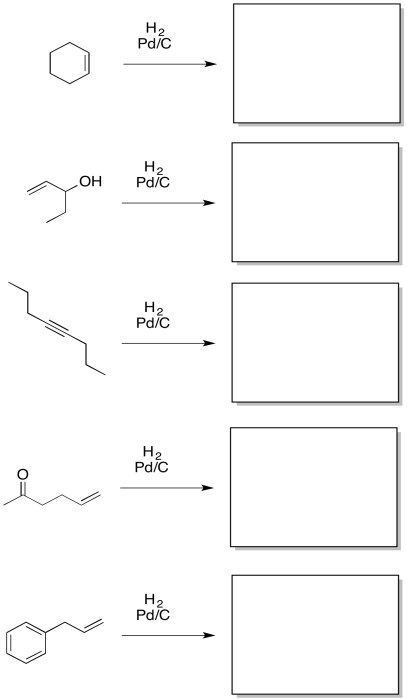
Problem OA5.2.
Provide products for the following reactions.
We can make the palladium catalyst even more selective by preparing it in a different way. Lindlar's catalyst is a very dramatic example of how reactivity can be tuned by using different compositions. To make Lindlar's catalyst, palladium is supported on calcium carbonate rather than carbon, together with other components, such as lead acetate and quinoline. These added components are the key to Lindlar's catalyst. They tune the reactivity so that the catalyst can react with alkynes but not with alkenes.
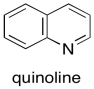
Figure OA5.8. Quinoline, used to poison a palladium catalyst so that it is less reactive.
As a result, if a compound is hydrogenated with a palladium catalyst in the presence of quinoline, an alkene is produced. Without the quinoline, you would get an alkane.
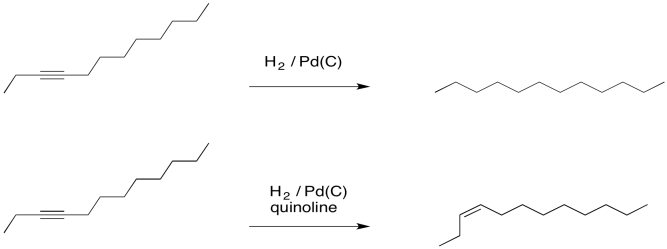
Figure OA5.9. Hydrogenation of an alkyne using Pd/C and Lindlar's catalyst.
Normally, if a catalyst hydrogenates an alkyne, no alkene is observed. That's because the alkene also reacts under the same conditions and is quickly converted to an alkane.
Not so fast, you say. If we just add one equivalent of hydrogen (that is, one molecule of hydrogen for every molecule of alkyne) then the reaction will stop after forming an alkene. That's very clever of you. However, you've missed a couple of important concepts. First of all, we are never dealing with individual molecules when we run a reaction; instead, we are dealing with vast numbers of molecules at a time. That means we will deal with statistical distributions. Maybe some molecules of hydrogen react with alkyne to produce alkene. Maybe some molecules go ahead and react with that alkene to produce alkane. Now, if we only added enough hydrogen for every alkyne to react with one H2, and some of them have already reacted with two, then somebody will be left out. There will be some leftover alkyne, too. That means we have made a mixture of alkyne, alkene and alkane.
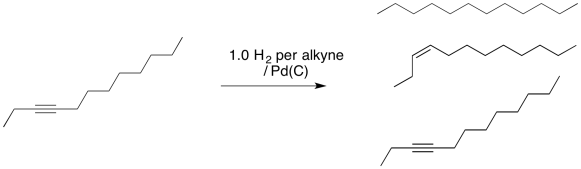
Figure OA5.10. An attempt to control hydrogenation using stoichiometry is seldom successful.
The other missing concept is that hydrogenation reactions usually run under a high pressure of hydrogen gas. That means many equivalents of hydrogen are needed in order to push the reaction forward. Regular monosubstituted alkenes may require several atmospheres of hydrogen gas. The reaction must be conducted in a heavy-walled vessel (possibly glass but more often steel) that can withstand these pressures. As noted already, something like benzene may require hundreds of atmospheres of hydrogen gas. There is generally way more hydrogen than there is alkene.
How does the presence of quinoline change the catalyst so dramatically? It seems that the answer is based on steric crowdedness. Although alkenes are flat, and don't seem very crowded, they may be crowded compared to an alkyne, which is linear. That difference makes alkynes even more reactive than alkenes with respect to hydrogenation.
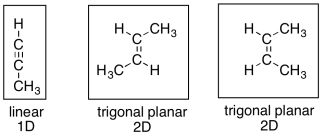
Figure OA5.11. A steric comparison of an alkyne with alkenes.
When these compounds bind to the surface of the catalyst, the alkyne takes up less space than the alkenes. It mostly lies along one dimension, whereas the alkenes are spread out into two dimensions.
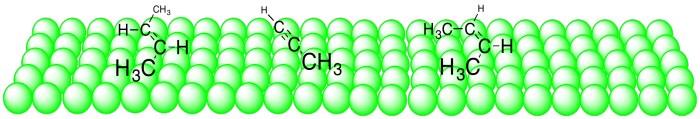
Figure OA5.12. Substrates binding to a catalyst surface.
The quinoline seems to simply take up space when it binds to the surface of the catalyst. As a result, the wider alkene can't bind as well as the narrow alkyne.
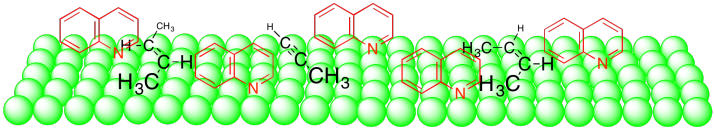
Figure OA5.13. Additives such as quinoline blocking the surface.
So it turns out that Lindlar's catalyst is a big deal. It provides a very selective reaction: production of an alkene from an alkyne. Furthermore, it doesn't just make any alkene. It only makes cis-alkenes. That's because the hydrogen atoms are both delivered from the surface of the metal. The alkyne binds to the surface of the metal and accepts the hydrogen atoms from that surface. As a result, both hydrogens end up on the same side of the new double bond. The alkene formed is then a cis-alkene.
The addition of two hydrogen atoms to the same side of the molecule is not limited to Lindlar's catalyst. It's a general feature of catalytic hydrogenation. As a result, catalytic hydrogenations are often diastereoselective; they result in the formation of one diastereomer, but not the other. Often, a mixture of two enantiomers forms. However, if the starting alkene already contained a chiral center, the alkene may bind preferentially to the catalyst using one face, and both hydrogens may be delivered to that face. That can lead to preferential formation of one enantiomer.
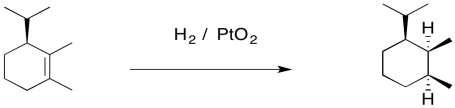
Figure OA5.13. Hydrogen atoms are added to the same face of the alkene.
Problem OA5.3.
Provide the missing reagents or products for the following reactions.
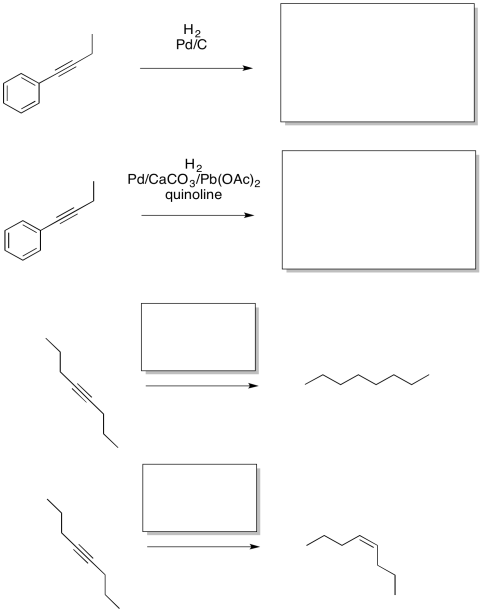
Hydrogenation catalysts are not always heterogeneous. Sometimes, they are soluble in the organic solvents used for hydrogenations. The most common one is Wilkinson's catalyst, (Ph3P)3RhCl. Wilkinson's catalyst, like Pd/C, is good at reacting with alkenes but leaving polar bonds alone. It is also highly selective, reacting only with the least sterically crowded alkenes. It only reacts with monosubstituted and disubstituted alkenes. If an alkene has fewer than two hydrogens attached to the double bond, Wilkinson's catalyst leaves it alone.
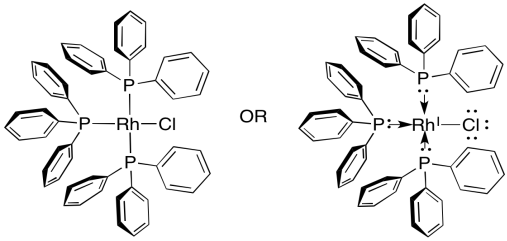
Figure OA5.14. Wilkinson's catalyst.
In contrast, Crabtree's catalyst, [(COD)(PCy3)(py)Ir]PF6 is a much more reactive catalyst. In part, that's because it is a more electrophilic, cationic catalyst; the PF6 is a non-reactive counterion. In addition, Crabtree's catalyst contains a sacrificial alkene ligand. COD is cyclooctadiene, a bidentate ligand that contains two double bonds. What happens to that ligand when the catalyst is exposed to hydrogen? It gets hydrogenated, of course. Without double bonds, it can no longer be a ligand. That leaves the catalyst with two open coordination sites, although really these sites are occupied by solvent molecules. Nevertheless, the solvent molecules bind only loosely, and can easily leave to make room for an alkene.
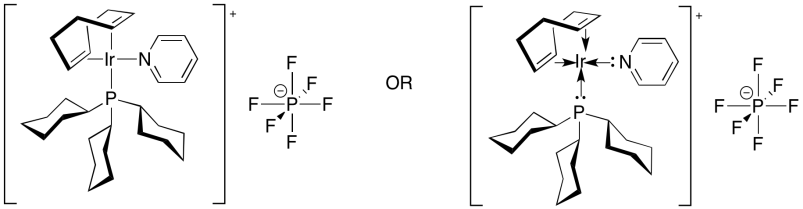
Figure OA5.15. Crabtree's (more reactive) catalyst.
As a result, Crabtree's catalyst is much less sensitive to steric crowding. Unlike Wilkinson's catalyst, it is perfectly capable of hydrogenating trisubstituted or even tetrasubstituted alkenes.
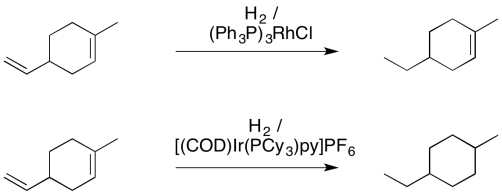
Figure OA5.16. The different hydrogenating ability of Crabtree's vs Wilkinson's catalyst.
The selectivity of these catalysts can be summarized as follows:
| Catalyst | Substrates |
| Lindlar's | alkynes |
| Wilkinson's | alkynes, mono- and disubstituted alkenes |
| Pd | alkynes, mono- and disubstituted alkenes |
| Crabtree's | alkynes, mono-, di-, tri- and tetrasubstituted alkenes |
| PtO2 | alkynes, mono-, di-, tri- and tetrasubstituted alkenes, benzene (high pressure) |
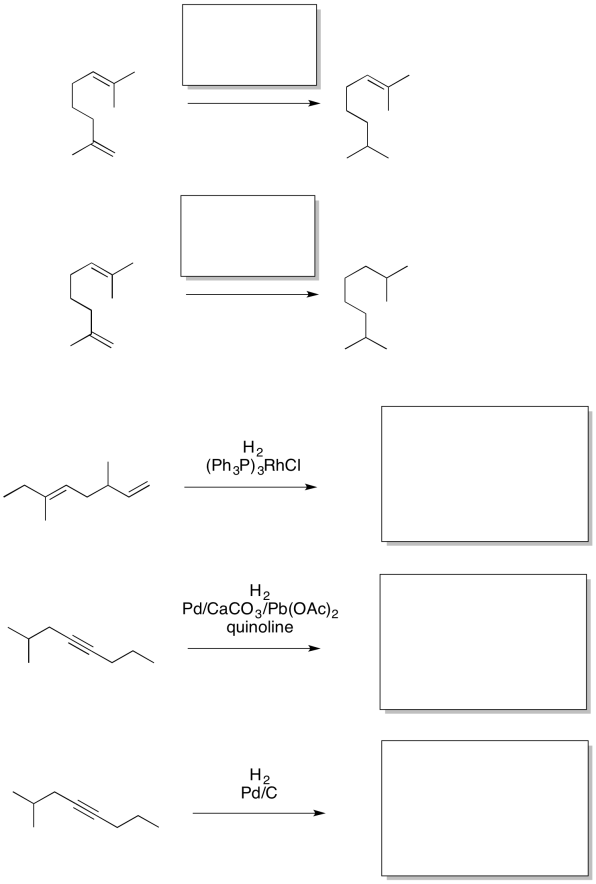
Problem OA5.4.
Provide the missing reagents or products in the following reactions.
Problem OA5.5.
Provide products for the following reactions.
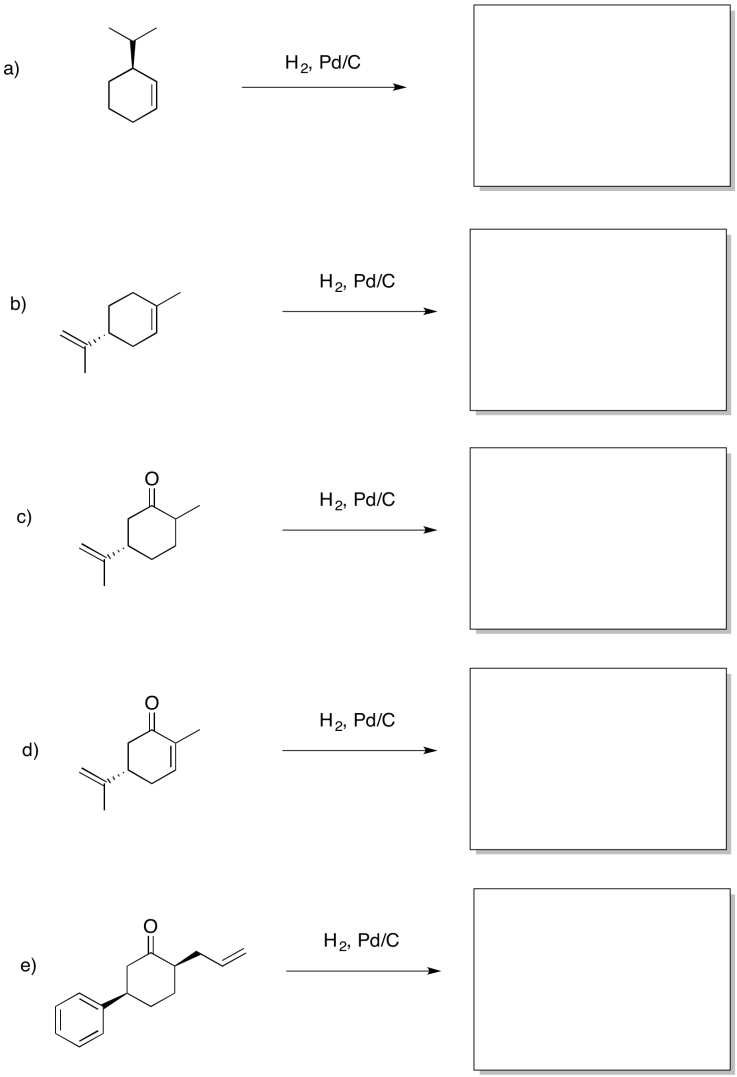
Problem OA5.6.
Provide products for the following reactions.
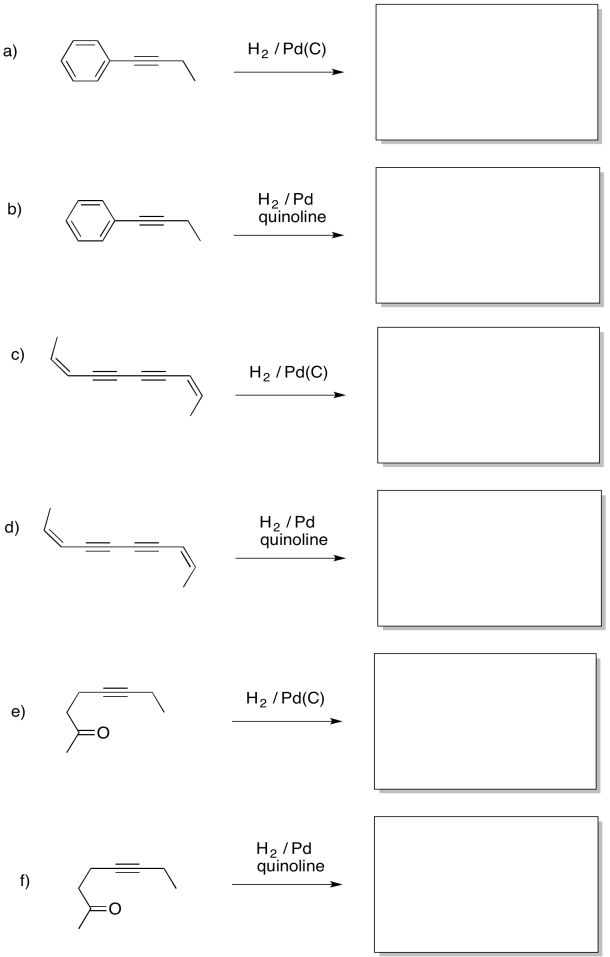
Problem OA5.7.
Provide reagents for the following reactions.
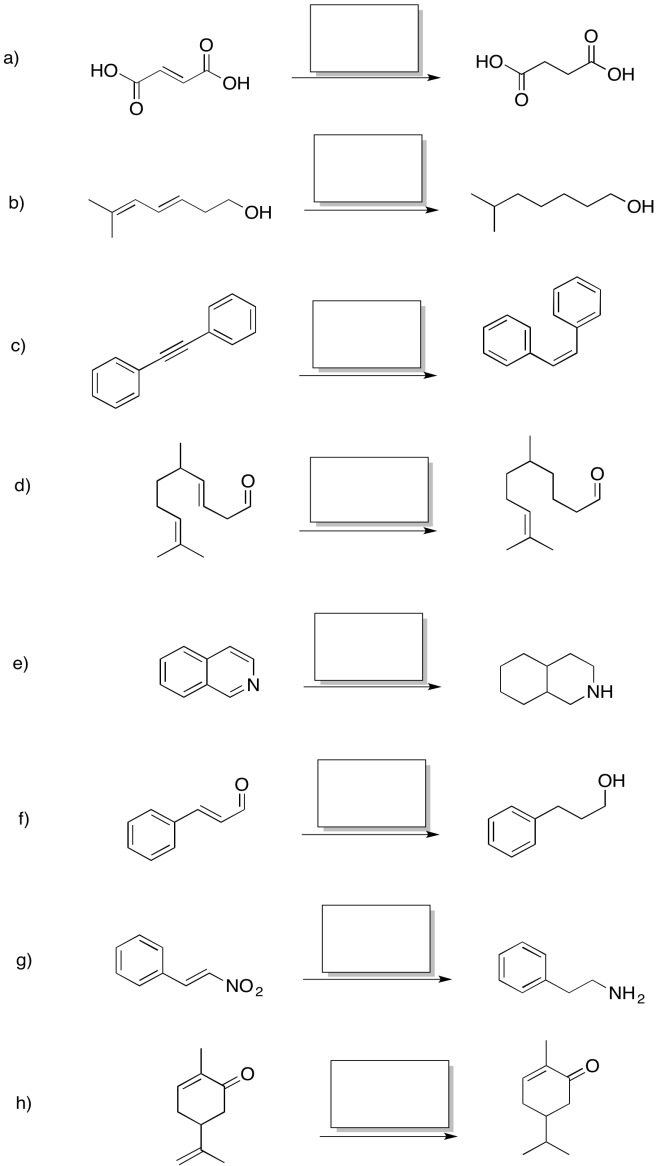
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Oxidative Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry