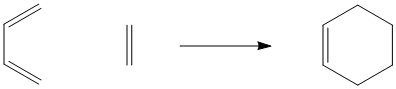
Figure OC3.1. The Diels Alder reaction.
Reactivity in Chemistry
Reactions Under Orbital Control
OC3. The Diels Alder Reaction
As noted previously, the Diels Alder reaction is a classic example of a cycloaddition reaction. A cycloaddition reaction has a lot in common with a pericyclic reaction like a Cope rearrangement.
Figure OC3.1. The Diels Alder reaction.
Unlike the Cope and Claisen rearrangements, this reaction often occurs intermolecularly (between two molecules). It requires an alkene on one molecule and a conjugated diene on the other molecule. The alkene is referred to as a "dienophile"; it reacts with the conjugated pair of double bonds. In the drawing below, the ethene and the 1,3-butadiene are labelled as diene and dienophile, respectively.
Figure OC3.2. The diene and dienophile in the Diels Alder reaction.
Problem OC3.1.
Draw curved arrows to keep track of electrons in the Diels Alder reaction.
Problem OC3.2.
Draw the aromatic transition state of the Diels Alder reaction.
Problem OC3.3.
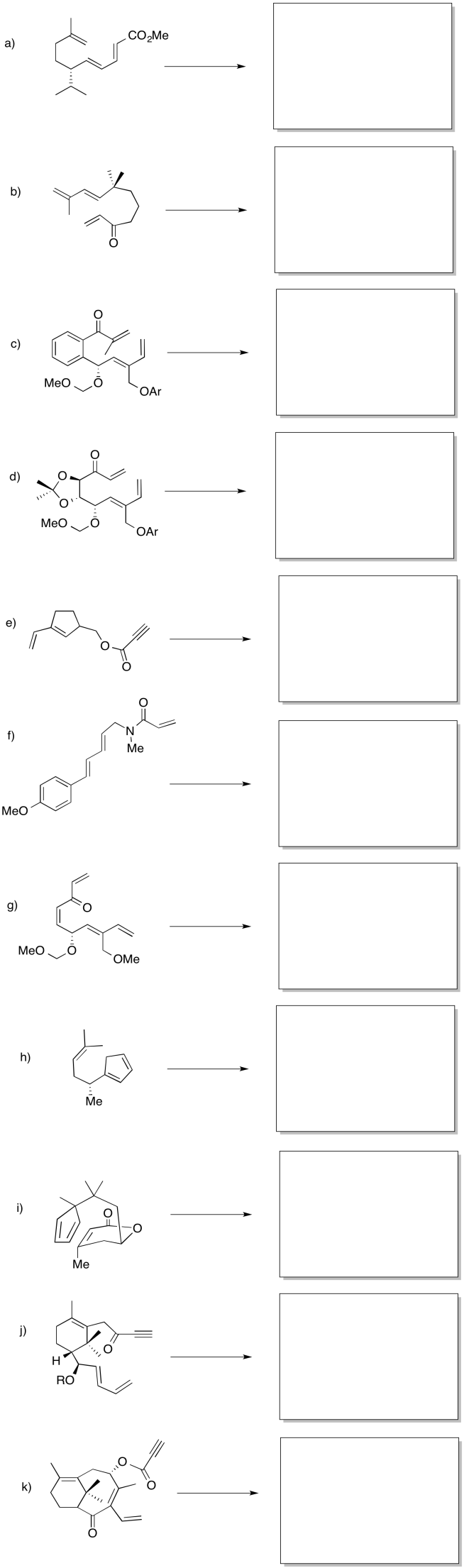
Provide products for the Diels Alder reactions below.
Although Diels Alder reactions frequently occur between two molecules, they can happen intramolecularly as well. In these cases, there must be an alkene in one part of the molecule that is able to reach over and interact with a conjugated diene on another part. In some ways, intramolecular Diels Alder reactions can be easier than their intermolecular counterparts for reasons of internal entropy. Rather than bringing two molecules together and combining them into one, the intramolecular Diels Alder starts with one molecule to begin with.
Problem OC3.4.
Many pericyclic reactions are reversible. The reversible process is usually named the same way as the forward reaction, but with the prefix "retro". For example, a Diels Alder reaction and the corresponding retro-Diels Alder reaction are shown together below.
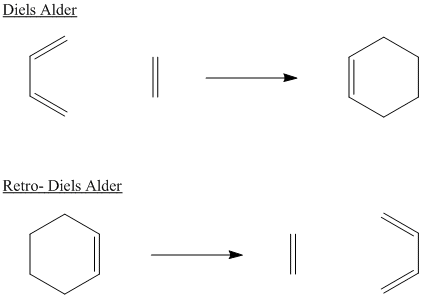
Figure OC3.3. A Diels Alder reaction and its reverse.
Typically, Diels Alder reactions occur at low or moderate temperatures (between 25 °C and 100 °C is a common range). In comparison, retro Diels Alder reactions require more elevated temperatures, often above 200 °C. The forward reaction is favoured at low temperature, whereas the retro reaction is favoured at high temperature.
Problem OC3.5.
Draw curved arrows for the retro-Diels Alder reaction to form ethene and 1,3-butadiene.
Problem OC3.6.
Explain why higher temperatures promote the retro-Diels Alder reaction using the expression for free energy of a reaction,
ΔG = ΔH - T ΔS.
Problem OC3.7.
Provide products for the following retro-Diels Alder reactions.
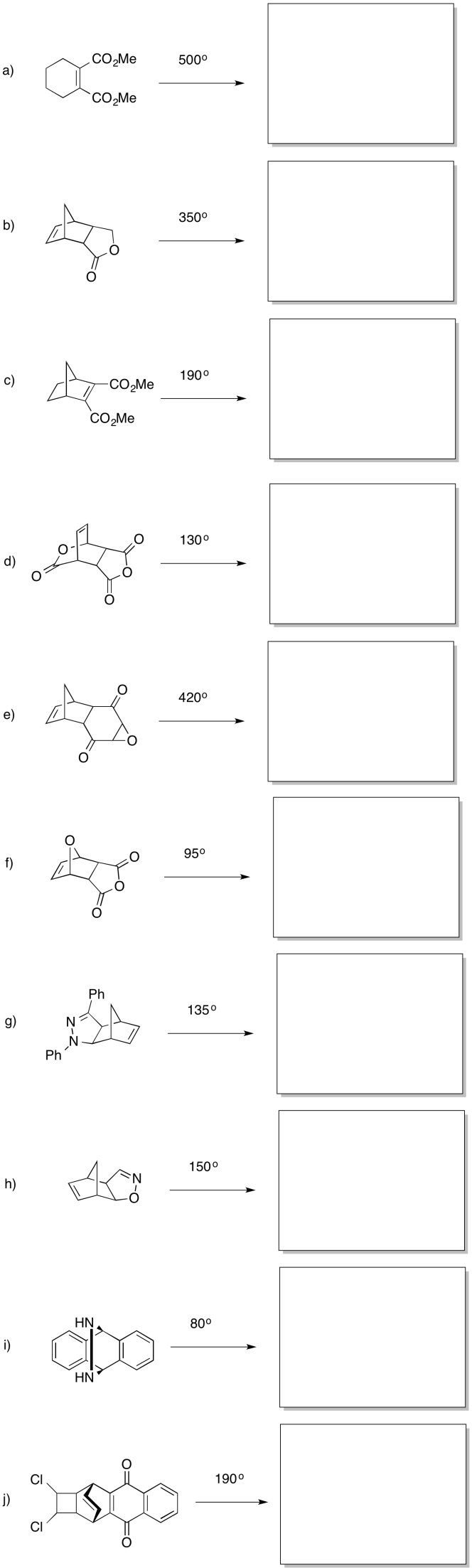
Problem OC3.8.
Sometimes, there are subsequent steps that occur after a cycloaddition reaction. For example, the following Diels Alder reaction involving naphthol produces a tautomerised product. Show the mechanism for the reaction sequence.
The reaction can be thought of in terms of a reorganization of electrons between these two molecules. In the Diels Alder reaction, we can think of an interaction between the LUMO on one molecule and the HOMO on the other. As it happens, the LUMO on one molecule has the correct symmetry such that it can overlap and form a bonding interaction with the HOMO on the other molecule.
Figure OC3.3. Qualitative molecular orbital picture of the Diels Alder reaction.
Pay attention to the p orbital drawings on the carbons that will bond to each other to form the six-membered ring. It is important that those orbitals are able to overlap with each other to form an in-phase interaction. In that way, these carbon atoms at the ends of the diene and dienophile are able to bond with each other.
A Diels Alder reaction is sometimes called a [2+4] addition reaction. A 2-carbon unit on one molecule interacts with a 4-carbon unit on another molecule.
In contrast, the addition of one regular alkene to another regular alkene would be called a [2+2] addition reaction. If this reaction occurred, two alkenes would come together to form a four-membered ring.

Figure OC3.4. A [2+2] addition reaction.
However, [2+2] addition reactions don't occur without special circumstances. We will look at the requirements for that reaction later.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: