Reactivity in Chemistry
Reactions Under Orbital Control
OC4. Regiochemistry in Diels Alder Reactions
The Diels Alder reaction is the most common cycloaddition reaction. It allows the construction of six-membered rings, These rings are very common in biological small molecules. These compounds are often of interest in medicinal chemistry and other areas of chemical biology. As a result, they are frequently targeted in synthetic studies. Being able to make these compounds, as well as compounds that are structurally related, is a key part of carrying out studies that will help us understand how they work in nature.
When two compounds combine in a Diels Alder reaction, a new ring is formed. Each reactant has two ends: there are two ends on the double bond in the dienophiles, and two ends on the diene, as well. Which end of the diene connects to which end of the dienophile? That is a question of regiochemistry, because we are asking where the reaction happens, or where the new bonds form.
We have thought about pericyclic reactions as being very different from polar reactions involving electrophiles and nucleophiles. That isn't always the case. In fact, there can be a pronounced polar component to these reactions. The cycloaddition of polar molecules is often much faster than nonpolar ones, and can be accelerated in the presence of Lewis acids. The Lewis acids can act as catalysts that activate the reactants, making them even more polar so that the attraction between the two components is greater.
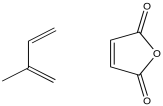
Problem OC4.1.
Maleic anhydride is frequently used as a dienophile in Diels Alder reactions. Explain how this component would enhance the interaction between the diene and the dienophile in the following case.

Problem OC4.2.
Show how, in the previous question, the interaction between the molecules would be enhanced through the addition of a Lewis acid, such as titanium tetrahloride, TiCl4.
If the reactants are polar, they may not be very symmetric. There may be a polar group attached to a double bond, causing the double bond to become more reactive but also making the molecule more complicated.
As a result, regiochemistry matters. If each component, the diene and the dienophile, have two very distinctive ends, then it matters which end connects to which, because we could potentially have two different products with completely different properties.
Generally, an understanding of resonance structures can help us to predict how two compounds are going to combine in a Diels Alder reaction.
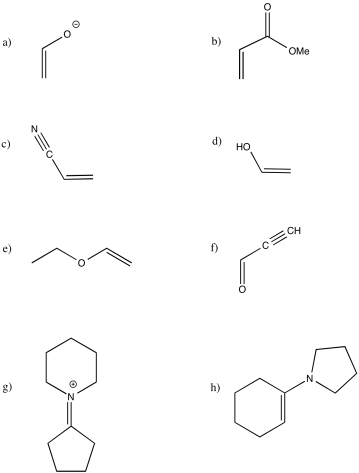
Problem OC4.3.
Identify each of the following compounds as electrophilic or nucleophilic at carbon. In each case, include an additional resonance structure to justify your choice.
If a diene and a dienophile are to react together, their interaction can be enhanced if one of the reactants is an electrophile and one is a nucleophile. Typically, in these cases the diene is designed to act as a nucleophile and the dienophile is chosen to act as an electrophile. However, there is no particular reason it has to be that way, other than the idea that the two double bonds of the diene already appear to be more electron-rich than the single double bond of the dienophile, and so it seems we only need to give them a little nudge in that direction.
In general, an electron-withdrawing group is installed on the dienophile. Remember, an electron-withdrawing group typically contains a multiple bond to an electronegative atom. If you think of electrophilic aromatic substitutions, these were the groups that typically acted as deactivating groups and meta-directors in that situation. Carbonyls, nitriles, and nitro groups are all examples of electron-withdrawing groups.
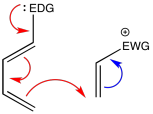
Figure OC4.1. Electron-donating and electron-withdrawing effects in a diene and a dienophile.
In a complementary manner, an electron-donating group is installed on the diene. Remember, π-donors are good examples of electron-donating groups. In electrophilic aromatic substitutions, electron-donating groups typically acted as ortho-para directors, and were usually activating groups in that situation. Alkoxyl groups and amines are good examples.
Problem OC4.4.
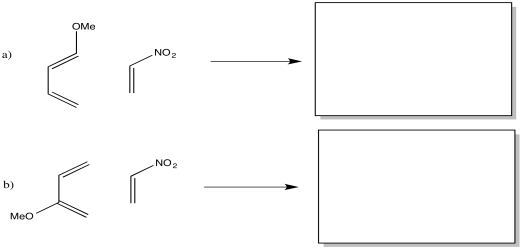
Illustrate this electrophile/nucleophile interaction using the diene, CH3OCH=CHCH=CH2 and the dienophile, CH2=CHNO2.
Problem OC4.5.
A similar interaction is possible using an isomer of the diene in the previous question, CH2=C(OCH3)CH=CH2. Illustrate the electrophile/nucleophile interaction between this diene and the dienophile, CH2=CHNO2.
Problem OC4.6.
Provide the products of Diels Alder reactions between the reactants below.
Perhaps the most widely used polar diene is Danishefsky's diene, developed in the laboratory of Samuel Danishefsky of Columbia University and Memorial Sloan-Kettering Cancer Center. Columbia University is a world leader in research in the field of organic chemistry. Danishefsky's lab specializes in the synthesis of complex molecules that may be of medicinal interest.
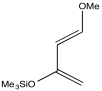
Figure OC4.2. Danishefsky's diene.
Danishefsky's diene, and others like it, allow for controlled regiochemistry in Diels Alder reactions because π-donation from the alkoxy and siloxy groups both push electron density toward the same location on the diene. It is then able to react more easily with a dienophile. If the dienophile is asymmetric, which in this case means one end is more positive than the other, then the reaction occurs in a predictable way.
Problem OC4.7.
Provide a mechanism for the formation of Danishefsky's diene under the following conditions.
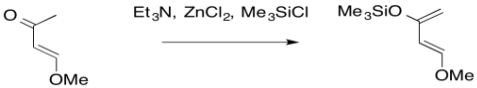
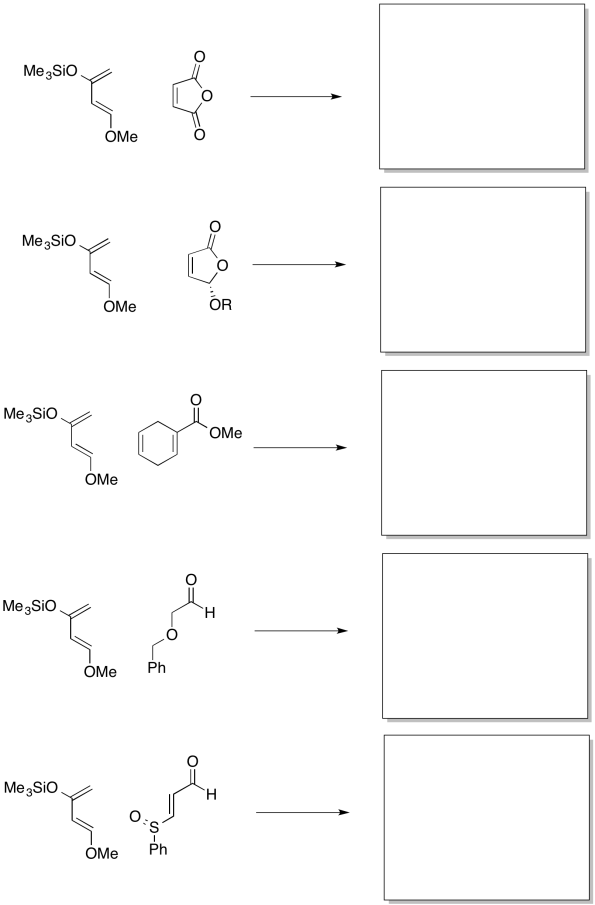
Problem OC4.8.
Show the products of the following reactions, including preferred regiochemistry.
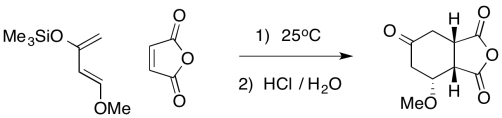
Problem OC4.9.
One of the reasons Danishefsky's is useful is that the silyl ether can be converted easily into a ketone. Provide mechanisms for the sequence below.
Sometimes, the Diels Alder reaction can work equally well with compounds other than alkenes. Of course, they work with alkynes instead of alkenes in the dienophile; the only difference in that case is that an extra double bond remains in the product. Sometimes, alkynes can react in the presence of alkenes, probably because the alkyne is less sterically hindered.
Carbonyls and imines also have double bonds. As such, they can also react sometimes. Because they are electrophilic by nature, they will react better with a more nucleophilic partner. They may also need to be activated.
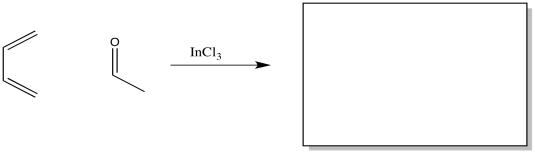
Problem OC4.10.
Show the product of the following reaction.
Note that, so far, we have looked at polarity-assisted Diels Alder reactions in which the diene takes the role of nucleophile (it is more electron-rich to begin with) and the dienophile takes the role of electrophile. It doesn't have to be that way. By placing an electron-withdrawing group on the diene and an electron-donating group on the dienophile, we would still get complementary reactivity. This type of approach is called an "inverse electron demand" DIels Alder reaction, because it is the opposite of the usual method.
Problem OC4.11.
Show a mechanism with curved arrows to make a prediction about regiochemistry in the following reaction .
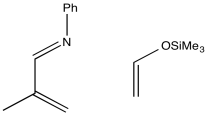
Problem OC4.12.
Show the products of the following reactions, including preferred regiochemistry.
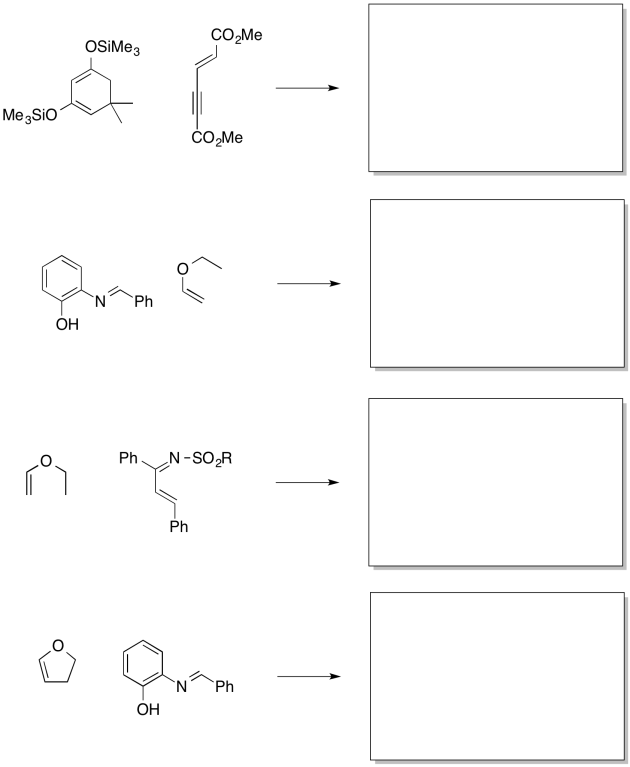
Problem OC4.13.
Show why the dienophile, 1,2-dimethoxybuta-1,3-diene, would be less successful as a regioselective nucleophile.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.