Reactivity in Chemistry
Reactions Under Orbital Control
OC5. Endo and Exo Products
The Diels Alder reaction is probably the most common cycloaddition. It allows the construction of six-membered rings, which are very common in biological small molecules which are frequently synthetic targets.
Often, there are already rings in the molecules undergoing reaction, and a new one is being added. When two cyclic structures combine in a Diels Alder reaction, a third ring is formed in between the original ones. There are different ways the two original rings can combine, leading to different stereochemical outcomes. These two outcomes are called "exo" and "endo" addition.
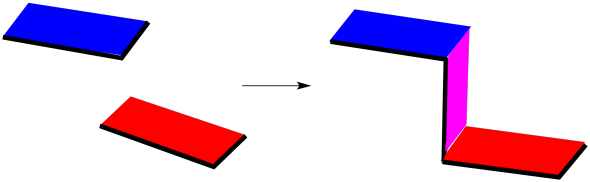
An exo addition looks something like this, schematically. The two molecules approach with minimal overall between their faces; they combine edge-on. The exo product that results has a sort of Z-shape.

Figure OC5.1. A drawing representing the orientation of reactants leading to an unfavoured "exo" Diels Alder product.
An endo addition looks more like this, schematically. Two cyclic molecules approach each other so that there is maximum overlap between their faces. The endo product that results has a kind of C-shape.
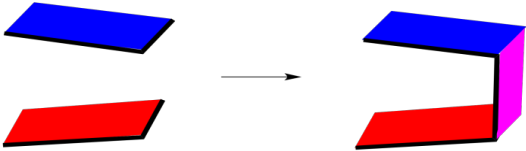
Figure OC5.2. A drawing representing the orientation of reactants leading to a favoured "endo" Diels Alder reaction.
Let's look at these two modes of addition with real molecules. Here we are adding furan, the diene, to maleic anhydride, the dienophile. The two reactants can approach each other such that one appears to be trailing behind the other, and in this case they appear to be facing the same direction, as far as the orientation of the oxygen atoms goes. This approach leads to the zig-zag exo product. In the other case, the two molecules can be directly on top of each other; one molecule appears to be folded underneath the other. This approach leads to the curled-up endo product.
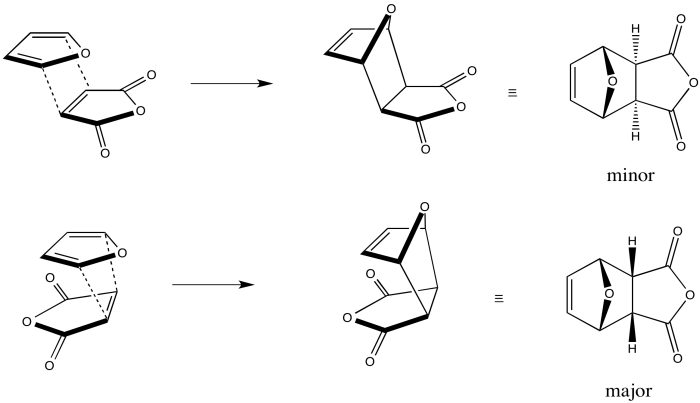
Figure OC5.3. A typical diene and dienophile undergoing exo addition (top) and endo addition (bottom).
In fact, as the diagram shows, the endo product is usually the favoured one. Often, the margin is substantial; one might see 90% endo product or greater in some cases, although the ratio is sometimes much lower.
The reason for this difference has something to do with an interaction between the delocalised π-system of the diene and the substituent groups attached to the dienophile. A number of researchers attribute the preference to a "secondary molecular orbital interaction" between the diene and the dienophile, whereas others describe the interaction as a London dispersion interaction, in which the weak intermolecular attractions stabilise the transition state in one geometry.
The endo and exo products are really two different diastereomers. If you think about it, you can see that when two rings fuse together to make a third, four new stereocenters can be created.
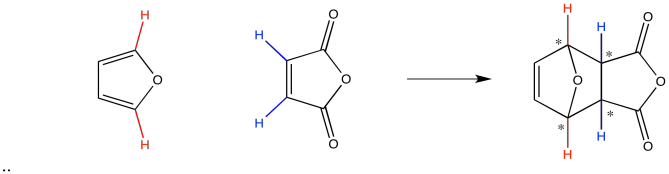
Figure OC5.4. A typical diene and dienophile undergoing Diels Alder cycloaddition, emphasizing the potential formation of four new chiral centers.
Since each chiral centre could have two possible configurations, there are sixteen possible stereoisomers that could result in the reaction shown above (24 = 16). That's a lot of structures. Just eight of them are shown below. Note that they occur in pairs of enantiomers. However, most of those diastereomers don't really occur, for reasons we'll consider in a moment. Before we even get that far, you may have already seen that the "enantiomeric pairs" on the left side of the picture aren't enantiomers at all, even though it looks like they are exactly opposite of each other. That's because these are meso compounds. This particular product contains a mirror plane of symmetry, so what looks like the enantiomer on paper is just the same thing turned over.
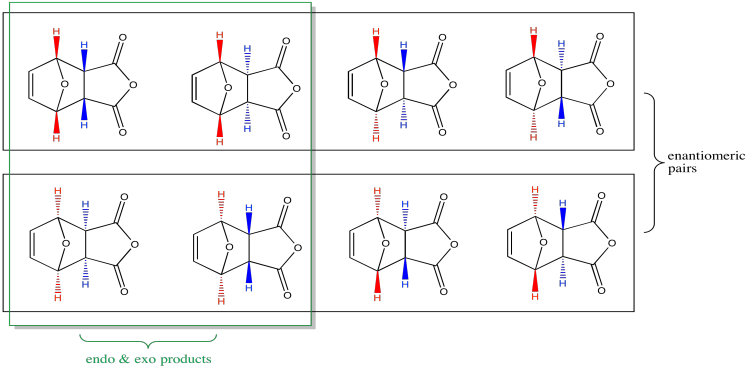
Figure OC5.5. Some possible stereoisomers in a Diels Alder cycloaddition.
Problem OC5.1.
Draw the other stereoisomers of the product formed from the reaction between furan and maleic anhydride, assuming any combination of stereochemical configurations could result.
If we look at the molecule in this way, with the hydrogens highlighted on the ends of the diene and the dienophile, it may be easier to see the stereochemical relationships in the exo and endo products. In the exo product, the pair of hydrogens on the diene ends up cis to the pair of hydrogens on the dienophile when the rings become fused. In the endo product, the opposite is true: the pair of hydrogens on the diene come out trans to the pair of hydrogens on the dienophile when the rings fuse together.
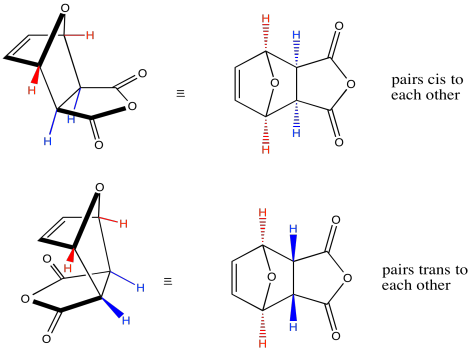
Figure OC5.5. Some possible stereoisomers in a Diels Alder cycloaddition: another look at endo and exo products.
Note that the hydrogens on the ends of the diene come out cis to each other, as well. They would be cis to each other in the ring, and that relationship does not change over the course of the reaction.
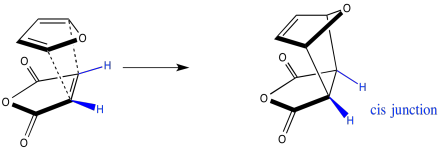
Figure OC5.6. Conservation of stereochemistry during a Diels Alder cycloaddition: A cis-relationship in the alkene leads to a cis-relationship in the ring.
The same thing is true with the hydrogens on the dienophile. Although we can't really describe the highlighted hydrogens as cis in the diene, they both lie on the same side of the line between the two carbons to which they are attached. They are both tilted toward the oxygen end of the molecule.
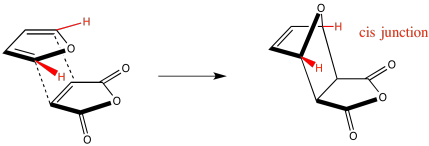
Figure OC5.7. Conservation of stereochemistry during a Diels Alder cycloaddition: A close spatial relationship in the diene leads to a cis-relationship in the ring.
It would be very difficult for the two cis hydrogens on one ring to become trans in the product, because it would require that one ring react via two different faces at the same time. It would be difficult to get one ring twisted around to do that. As a result, we can ignore most of the diastereoisomers that could result from a Diels Alder reaction on paper, and focus on the endo and exo products.
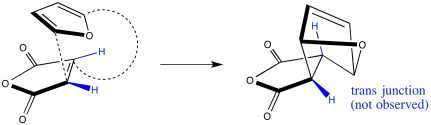
Figure OC5.8. A lack of conservation of stereochemistry during a Diels Alder cycloaddition: This is just wrong.
Of course, Diels Alder reactions between rings like these always have the potential to produce pairs of enantiomers. Because two enantiomers are equal in energy to each other, there is no inherent preference for forming one over the other. In other words, there would actually be two endo products, and they would be enantiomers of each other. There would also be two exo products, and those would be enantiomers of each other, too.
Methods are available for influencing the formation of one enantiomer exclusively. These methods involve the use of chiral catalysts. These catalysts may contain Lewis acidic metals or they may contain some combination of hydrogen bond donors and acceptors. In either case, the role of the catalyst is to tether the reactants close together so that they will undergo the reaction. If the catalyst is itself chiral, the reactants will often fit together one way more easily than another, so that the formation of one enantiomer is preferred.
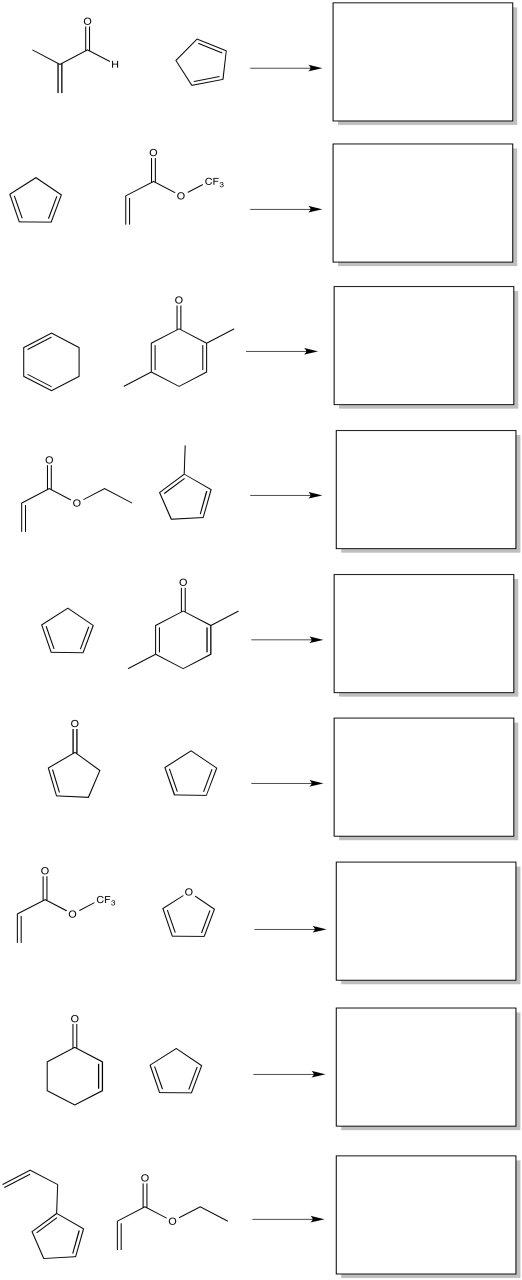
Problem OC5.2.
Show the products of the following reactions, including preferred stereochemistry.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: