
Reactivity in Chemistry
Reactions Under Orbital Control
OC7. Facial Selectivity
In addition to the consequences of endo- vs. exo- additions in the Diels Alder reaction, pericyclic reactions are subject to additional stereochemical constraints. In this section we will look at more issues of topology, or how the surfaces of the molecules fit together. Although this topic applies to both cycloadditions and sigmatropic rearrangements, we will start by looking at cycloadditions.
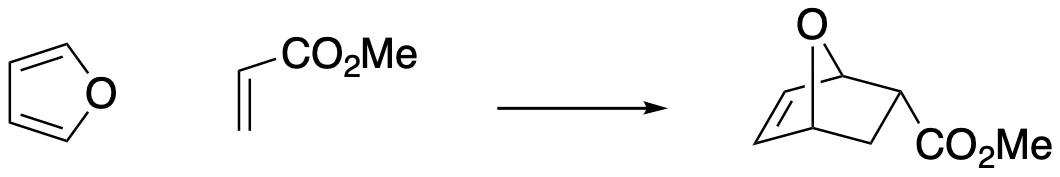
Figure OC7.1. The Diels Alder reaction is a typical cycloaddition.
So far, we have made the assumption that two molecules in a Diels Alder reaction would simply come together in a face-to-face fashion. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. In fact, it generally happens that way, and this relationship is called a "suprafacial" addition.

Figure OC7.2. An illustration of two planes (molecules) meeting in a suprafacial manner (left) compared to an antarafacial manner (right).
However, there are less common cases in which something else happens. Imagine one molecule is able to approach the other at a slight angle, such that it is able to slip in between the ends of the other molecule. A molecule would be interacting with its neighbour not just along one face, but along two. It would form bonds via both its top and its bottom face. Alternatively, if the two molecules have some flexibility, they could twist so that a molecule presents one face at one end of the chain and the other face at the other end of the chain. This type of addition is called "antarafacial". It's not exactly like the cartoon shown above, but to get additional detail we will need to look at molecular orbital pictures.
We haven't worried about antarafacial reaction in the Diels Alder reaction because it would be difficult to get two different faces of a small molecule like ethene to interact with another molecule at the same time. Maybe not impossible, but not likely. So when we thought about thermal vs. photochemical conditions, we assumed the "bottom" face of the butadiene (from the point of view shown in a picture) had to interact with the "top" face of the ethene, or vice versa. When we looked at it that way, we noticed that the orbitals at the end of each chain were just right to σ-bond with the orbitals on the other chain, forming a new ring. We assumed it would require an interaction between the HOMO of one molecule and the LUMO of another; these reactions may seem strange, but we still need to take electrons from one place and drop them in a hole somewhere else. Those orbitals match up perfectly.
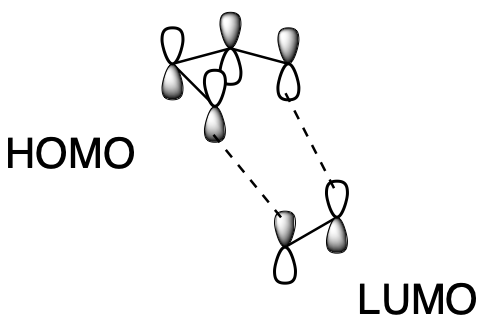
Figure OC7.3. Sigma bond formation in a cycloaddition reaction with 6 π electrons.
On the other hand, when one ethene molecule reacts with another ethene molecule, the HOMO one one molecule didn't line up that well with the LUMO on the other. It didn't look like these two molecules were ready to form sigma bonds with each other, at least not under normal, "thermal" conditions, in which any energy for a reaction just has to be provided by any heat present in the system.
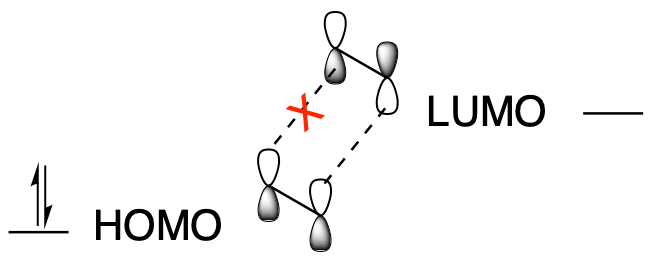
Figure OC7.4. Sigma bond formation in an electrocyclic reaction with 4 π electrons.
In that case, the reaction can't happen under thermal conditions. But if we add light, we are back in business. The light, if it's the right wavelength, excites an electron from the HOMO into the LUMO. Now the LUMO isn't the LUMO anymore. It's occupied, not unoccupied. It's become the new HOMO (some people call it a SOMO, for singly occupied molecular orbital, because it can behave differently than a HOMO with 2 electrons). All this excited state molecule has to do is find a second molecule that hasn't been excited. Its LUMO will still have the right symmetry to interact with that excited state HOMO. A ground state (unexcited) molecule will be easy for it to find because ony a small fraction of molecules will absorb photons and become excited at the same time.
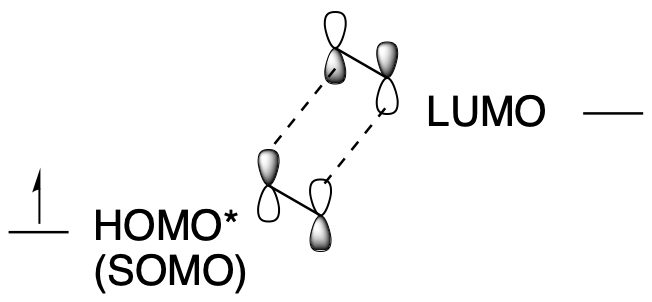
Figure OC7.5. Sigma bond formation in an electrocyclic reaction with 4 π electrons under photochemical conditions.
So that's the story for cycloadditions, in which the most common reactions are suprafacial. Other pericyclic reactions, electrocyclic and sigmatropic reactions, are more complicated because they can easily react with different topologies (spatial relationships). In electrocyclic and sigmatropic reactions, there is an interaction between different ends of a chain to produce a new σ-bond. For example, in an electrocyclic reaction, a conjugated system gives up a π-bond to form a new σ-bond and make a ring. The reaction can also happen in the opposite direction. This kind of reaction is called an electrocyclic reaction. In a sigmatropic rearrangement such as a Cope or Claisen rearrangement, there is a similar formation of a σ-bond between the ends of a chain.
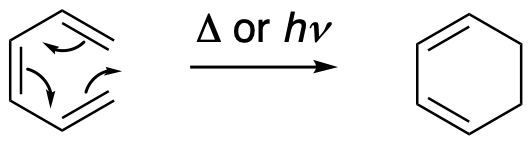
Figure OC7.6. An electrocyclic reaction.
When two ends of a molecule fold in to bond with each other, you can imagine doing so in either of two ways. Maybe the two ends roll towards each other, so that the top face on one end of the molecule connects with the top face on the other. Maybe the top face on one end connects with the lower face on the other end.
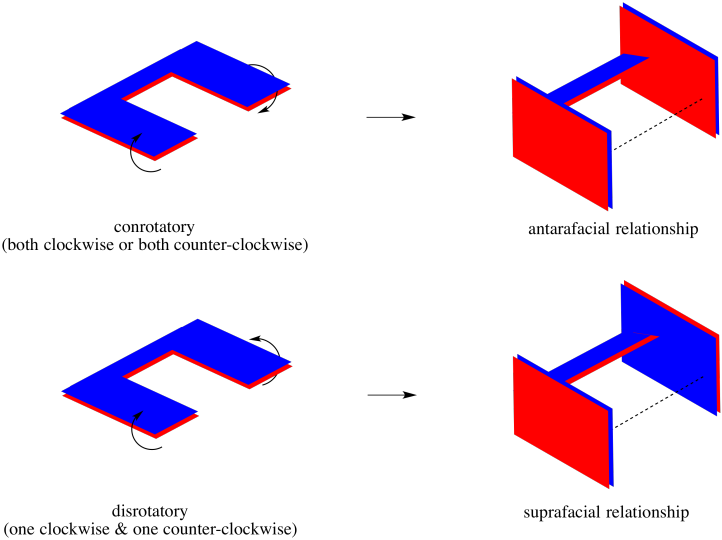
Figure OC7.7. An illustration of two ends of a chain twisting in a conrotatory manner (top) compared to a disrotatory manner (bottom).
In these kinds of reactions, that take place within a chain rather than between faces of different molecules, the motions that the molecule would undergo to put these interactions in place are called "conrotatory" (rotating together) and "disrotatory" (rotating opposite ways). The interactions they produce, however, are similar to the interactions in cycloadditions, with either an antarafacial or a suprafacial relationship, respectively.
Once again, we are going to take a look at σ-bond formation in these reactions. Again, we will need to think about orbital interactions. Let's start with an orbital picture that is slightly simpler than the hexatriene molecule in the electrocyclic reaction above. We'll start with a reaction involving the slightly smaller butadiene. This reaction doesn't look so easy because of the strained four-membered ring produced. Don't worry about that. All of these reactions are reversible, and the equilibrium in this one just happens to lie further to the left than most of the others we've seen.
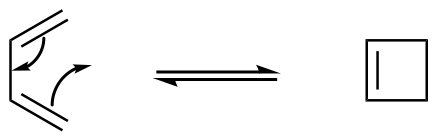
Figure OC7.8. Another electrocyclic reaction.
Using this example, we can more clearly see why we need to have the two ends of the molecule twist when they are forming the σ-bond. In butadiene, the four hydrogens at the ends of the chain are all in plane with each other because they are all attached to carbons in a conjugated system. In cyclobutene, The hydrogens have all twisted out of the plane of those carbons.
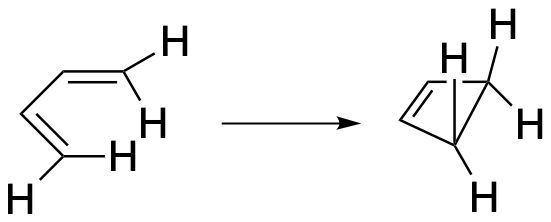
Figure OC7.9. Twisting to make the sigma bond in an electrocyclic reaction.
So here is the Huckel diagram for butadiene. Once again, a Huckel diagram just ignores everything but the π-bonding.
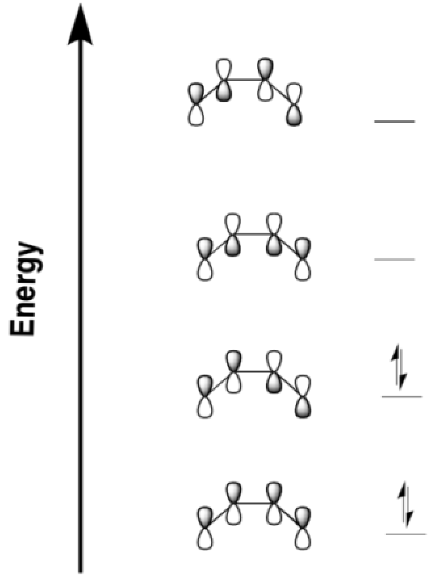
Figure OC7.10. The Huckel molecular orbital diagram for butadiene.
In an electrocyclic reaction, we only need to consider one orbital. Is it the HOMO or the LUMO? It doesn't make sense to consider the LUMO, because it doesn't have electrons in it. It's usually considered a virtual orbital, not a real one. It's only useful if we are trying to imagine a place where electrons could go, but where there are not electrons already. If we focus on σ-bond formation, then we want the orbitals on the two ends of the chain to form an in-phase combination.
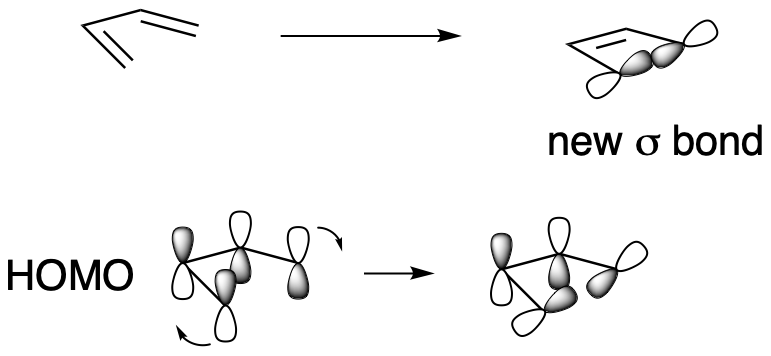
Figure OC7.11. Sigma bond formation in an electrocyclic reaction with 4 π electrons.
For that to happen, we are going to need one orbital to tilt in and the other to tilt out, because the lobes at the end of the chain are in opposite phase. They can do that if they both rotate the same direction: either both clockwise or both anticlockwise. This is a conrotatory reaction.
That's what happens under thermal conditions. Under photochemical conditions, we won't be dealing with that orbital because it won't be the HOMO anymore. The new HOMO will be the next level up in the diagram. In that case, the lobes at the two ends of the chain are in phase with each other. They can both tilt in or they can both tilt out to form the new σ-bond. That means one will move clockwise and one will move counterclockwise. This is a disrotatory reaction.
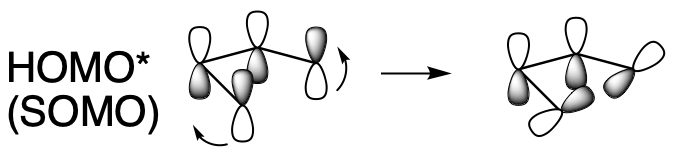
Figure OC7.12. Sigma bond formation in an electrocyclic reaction with 4 π electrons with light.
Now let's think about the other example we have of an electrocyclic reaction. That one involved hexatriene. The Huckel MO diagram for hexatriene is shown below.
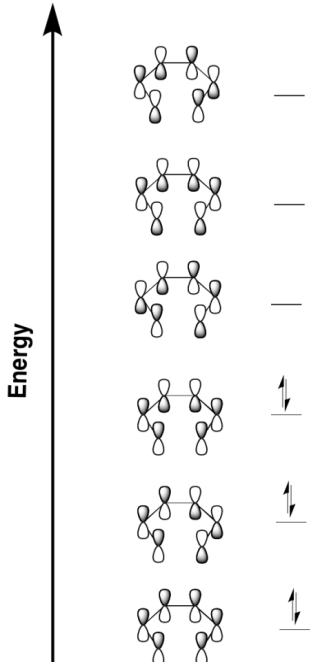
Figure OC7.13. The Huckel molecular orbital diagram for hexatriene.
This time, the LUMO that will be involved in σ-bond formation is very different from the one in butadiene. The lobes at either end of its chain are in phase with each other. So, they can both tilt in or they can both tilt out to get an in-phase, bonding interaction. One needs to spin clockwise but the other needs to spin counterclockwise. This is a disrotatory reaction.
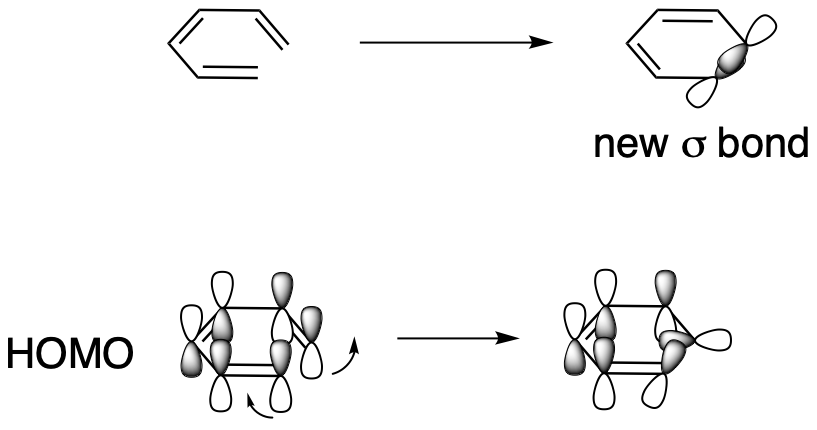
Figure OC7.14. Sigma bond formation in an electrocyclic reaction with 6 π electrons.
Again, under photolytic conditions, everything changes. The LUMO gets an electron and becomes the new HOMO. The lobes at either end are out of phase. One lobe needs to tilt in and the other lobe needs to tilt out so they can form an in-phase, bonding combination. To do that, they could both rotate sunwise, or they could both rotate widdershins, but they have to rotate in the same direction. That's conrotatory.
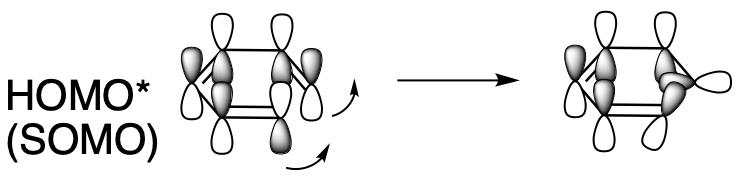
Figure OC7.15. Sigma bond formation in an electrocyclic reaction with 6 π electrons with light.
We can summarize how molecules can react together in cycloadditions and pericyclic reactions as shown in the table below.
Table OC7.1. Rules for pericyclic reactions.
| Number of electron pairs | suprafacial / disrotatory | antarafacial / conrotatory |
| even | photochemical | thermal |
| odd | thermal | photochemical |
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: