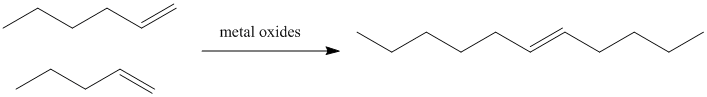
Figure OC10.1. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
Reactivity in Chemistry
Reactions Under Orbital Control
OC10. Olefin Metathesis
Olefin metathesis, or alkene metathesis, is an important process in petroleum refining and in the synthesis of important compounds such as pharmaceuticals. The mechanism of olefin metathesis is related to pericyclic reactions like Diels Alder and [2+2] reactions. In other words, it occurs through the concerted interaction of one molecule with another.
In petroleum refining, heating alkenes over metal oxide surfaces results in the formation of longer-chain alkenes. In particular, terminal olefins (with the double bond at the end of the chain) are converted into internal olefins (with the double bond somewhere in the middle of the chain).
Figure OC10.1. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
What does that reaction have to do with addition reactions involving double bonds?
Clearly, the alkenes have double bonds. In addition, so do the metal oxides. Metal atoms inside the metal oxides are bridged together by oxygen atoms. The surface of the metal oxides may be covered with a mixture of hydroxyl groups as well as terminal oxides (M=O groups). The terminal oxides on the surface are the important part of the catalyst.
Figure OC10.2. A simplified structure of a chunk of metal oxide surface.
When metal alkylidene complexes were developed in the 1970's, it was found that they, too, could catalyze this reaction.
Figure OC10.3. In olefin metathesis, the active species is a metal carbene.
In fact, scientists working in petroleum chemistry soon came to believe that metal oxides on the catalyst surface were converted to alkylidenes, which then carried out the work of olefin metathesis.
The reaction, it turns out, involves a [2+2] cycloaddition of an alkene to a metal alkylidene (to the metal-carbon double bond). This reaction results in a four-membered ring, called a metallacyclobutane.
The [2+2] cycloaddition is quickly followed by the reverse reaction, a retro-[2+2]. The metallacyclobutane pops open to form two new double bonds.
This mechanism is called the Chauvin mechanism, after its first proponent, Yves Chauvin of the French Petroleum Institute. Chauvin's proposal of this mechanism shortly after the discovery of metal alkylidenes by Dick Schrock at DuPont earned him a Nobel Prize in 2005. Chauvin and Schrock shared the prize with Bob Grubbs, who made it possible for the reaction to be adapted easily to the synthesis of complex molecules such as pharmaceuticals.
Figure OC10.4. The Chauvin mechanism for olefin metathesis.
Why does olefin metathesis lead to the formation of internal alkenes? The [2+2] addition and retro-[2+2] reactions occur in equilibrium with each other. Each time the metallacyclobutane forms, it can form two different pairs of double bonds through the retro reaction. In the presence of terminal alkenes, one of those pairs of alkenes will eventually include ethene. Since ethene is a gas, it is easily lost from the system, and equilibrium shifts to the right in the equation below.
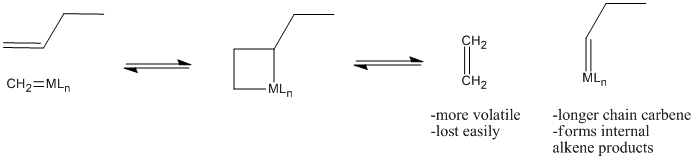
Figure OC10.5. Olefin metathesis proceeds via cycloaddition to produce metallocylcobutanes.
That leaves a longer-chain alkylidene on the metal, ready to be attached to another long chain through subsequent [2+2] addition and reversion reactions.
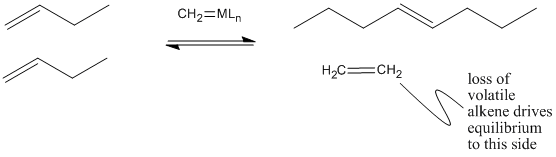
Figure OC10.6. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins" because volatile, short chain products are lost, shifting equilibrium.
In most cases, a [2+2] addition won't work unless photochemistry is involved, but it does work with metal alkylidenes. The reason for this exception is thought to involve the nature of the metal-carbon double bond. In contrast to an orbital picture for an alkene, an orbital picture for an alkylidene pi bond suggests orbital symmetry that can easily interact with the LUMO on an alkene. That's because a metal-carbon pi bond likely involves a d orbital on the metal, and the d orbital has lobes alternating in phase like a pi antibonding orbital.
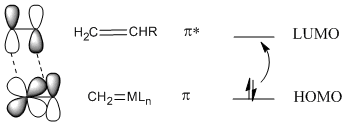
Figure OC10.7. Olefin metathesis produces longer-chain "internal olefins" from shorter chain "terminal olefins".
A variety of catalysts have been developed for olefin metathesis. The laboratories of Bob Grubbs at CalTech and Dick Schrock at MIT have been particularly important in this area, although other labs have contributed significantly, including that of Amir Hoveyda at Boston College. The Schrock catalysts are based on tungsten or, more commonly, molybdenum. These catalysts are among the most efficient available, operating at very high turnover frequencies.
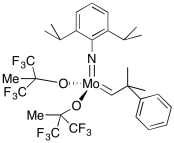
Figure OC10.8. A Schrock catalyst for olefin metathesis.
There are other variations, including some designed for alkyne metathesis rather than alkene metathesis. There is also a commercially-available Schrock-Hoveyda catalyst; that one is chiral, and can be used to carry out olefin metathesis enantioselectively. When presented with a racemic mixture of substrates, this catalysts will select one substrate preferentially over the other. However, the Schrock catalysts can be intolerant of heteroatom functional groups. Molybdenum and tungsten are highly electrophilic, so lone pairs on atoms such as halogens or nitrogens can sometimes inhibit these metals. These compounds are also highly intolerant of air and moisture, for similar reasons.
Grubbs catalysts are more tolerant than Schrock catalysts, although they do not operate at nearly the speed that the molybdenum and tungsten ones can achieve. Because they are less sensitive to air and moisture, they are more commonly used for small-scale, benchtop reactions. In larger scale, industrial reactions, air- and moisture-sensitivity is usually a less compelling factor than speed.
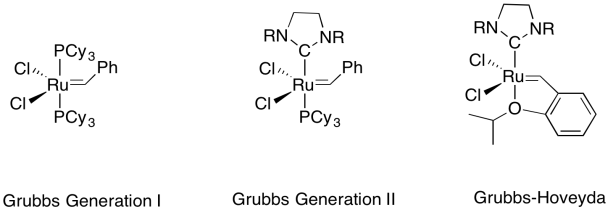
Figure OC10.9. A series of Grubbs catalysts for olefin metathesis.
There are several "generations" of Grubbs catalysts that are commercially available, but a range of others have also been developed. New generations tend to offer a much higher reaction rate.
In addition to the simple "partner-swapping" of olefin metathesis, which is very important to the petroleum industry, there are other useful variations on the reaction. For example, a cyclic alkene that undergoes olefin metathesis forms two new double bonds, but these parts of the molecule are still connected to each other. Thus, an olefin metathesis between a cyclic alkene a chain alkene might produce a diene.
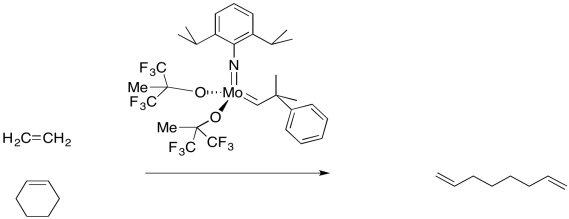
Figure OC10.10. An example of ring opening metathesis. Usually, the rings are more strained than this one.
But remember, these reactions occur in equilibrium. If a cyclic alkene can be converted into a diene, then under the right conditions, a diene can be converted to a cyclic alkene. This reaction has become very important in the synthesis of organic compounds by the agricultural and pharmaceutical industries. Rings of many different sizes, even very large ones, can be made in this way.
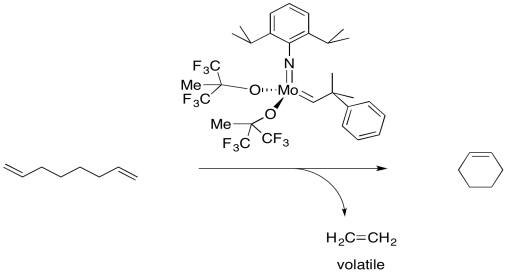
Figure OC10.11. An example of ring-closing metathesis.
On the other hand, if the cyclic alkene by itself is treated with an olefin metathesis catalyst, it may link to other cyclic alkenes. That's because each cyclic alkene forms two new double bonds, one on each end. If each molecule forms two double bonds, a long chain of dienes will form. The result would be a polymer.
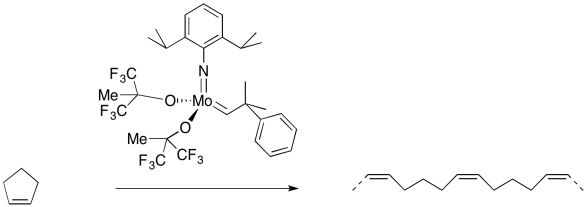
Figure OC10.12. An example of ring-opening metathesis polymerisation (ROMP).
Problem OC10.1.
Show the products of the following reaction.
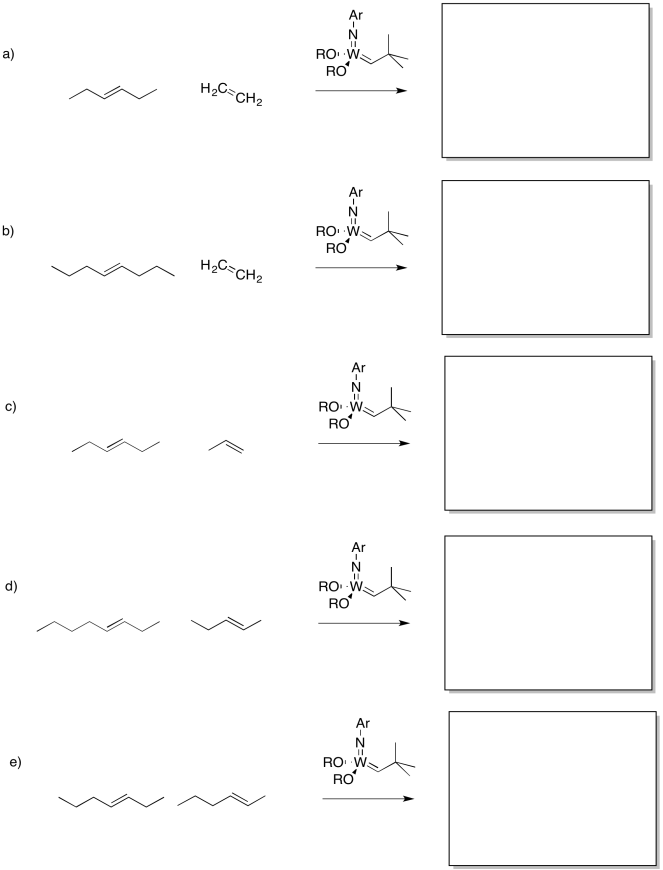
Problem OC10.2.
Show the products of the following reaction.
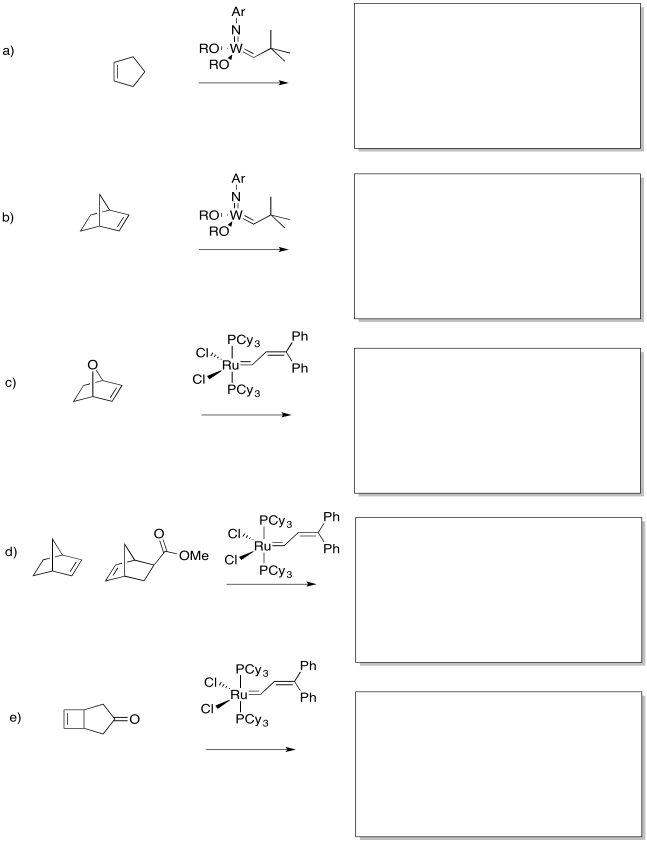
Problem OC10.3.
Show the products of the following reaction.
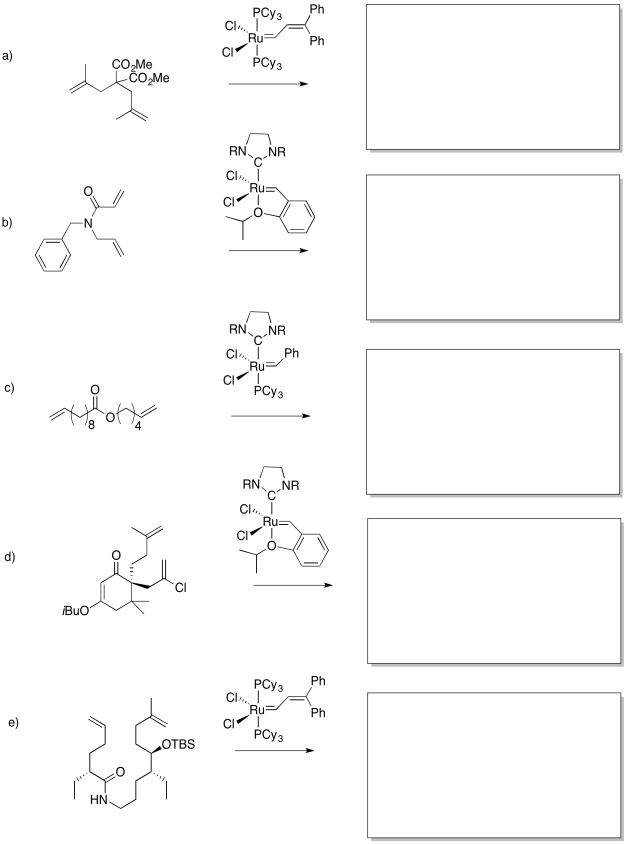
Problem OC10.4.
Fill in the blanks in the following synthesis.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: