Reactivity in Chemistry
Reactions Under Orbital Control
OC11. Solutions to Selected Problems
Problem OC1.1.

Problem OC1.2.
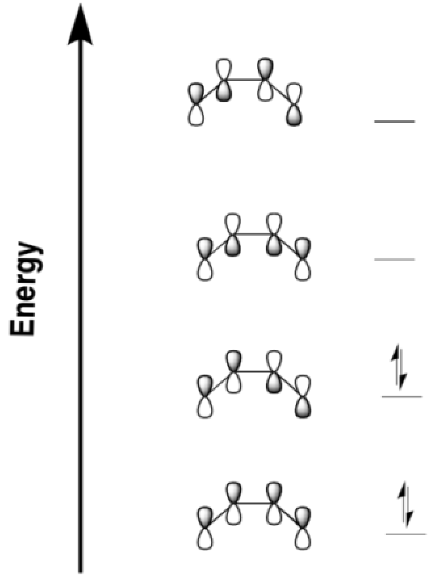
Problem OC2.1.
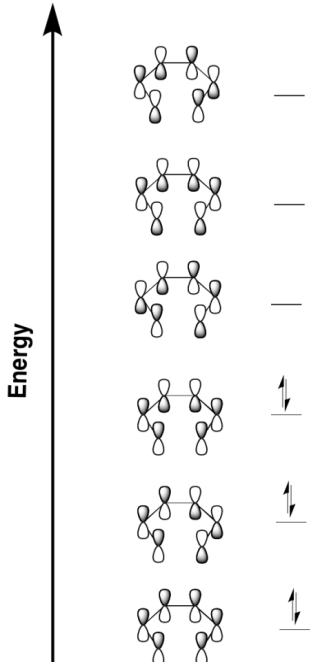
Problem OC2.2.
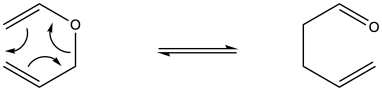
Problem OC2.3.
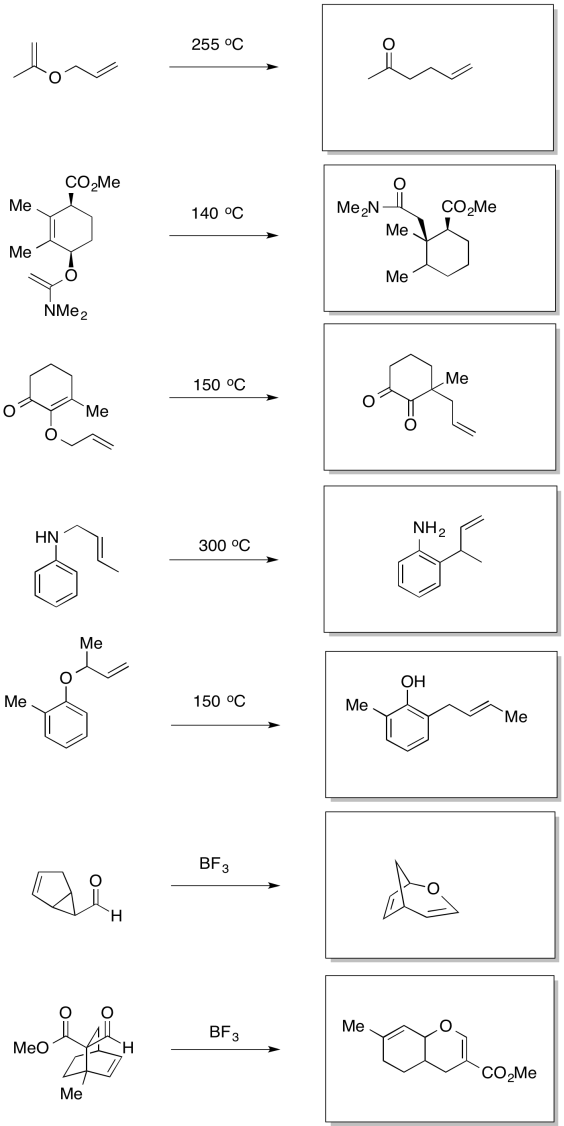
Problem OC3.1.
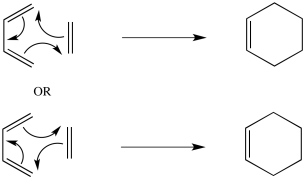
Problem OC3.2.
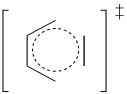
Problem OC3.3.
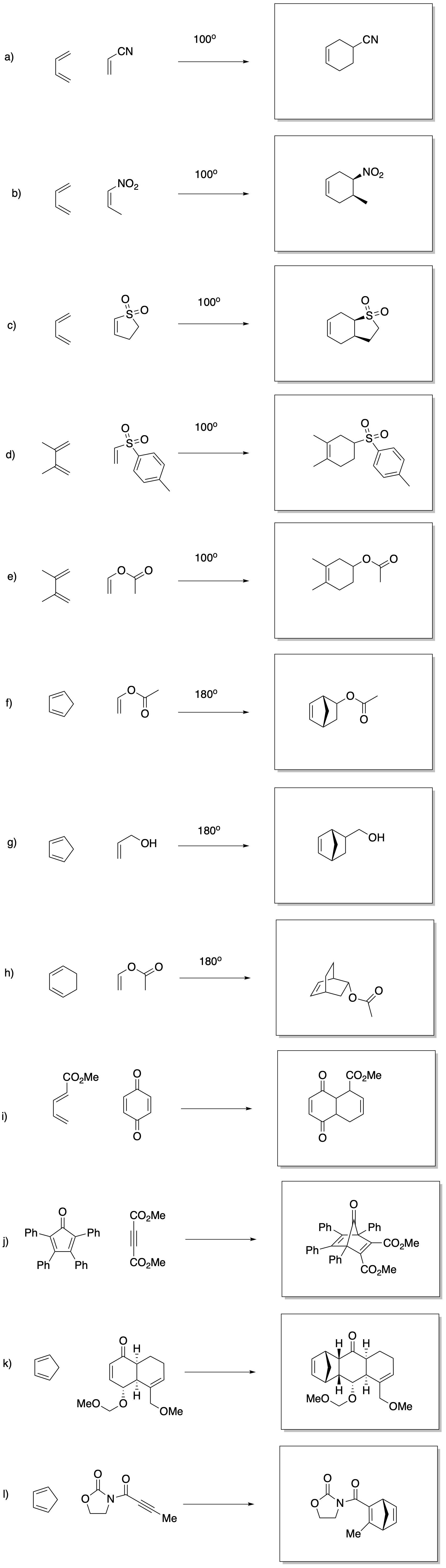
Problem OC3.4.
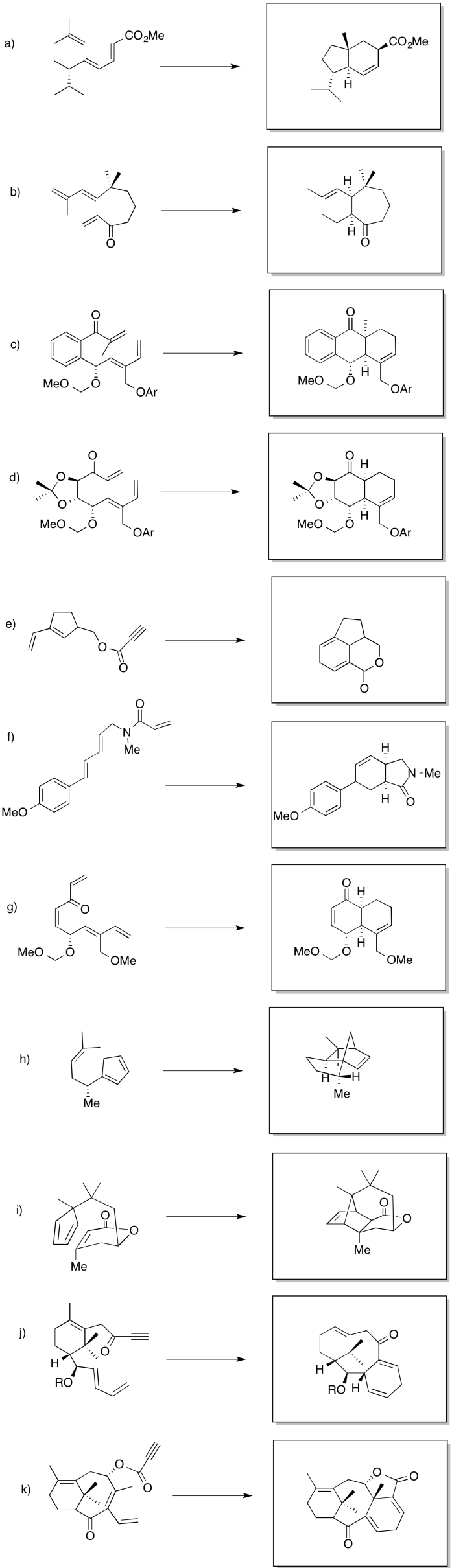
Problem OC3.5.
Problem OC3.6.
The retro-Diels Alder reaction is entropically favoured, because two molecules are made from one molecule. As a result, energy becomes partitioned into additional states because the degrees of freedom are increasing. Internal entropy increases during this reaction. Mathematically, the entropy term is negative in the expression for free energy, so as entropy increases the free energy becomes lower. Because that term is actually a product of entropy and temperature, an increase in temperature has the effect of amplifying the influence of entropy on the free energy of the reaction. Hence, this reaction is favoured by internal entropy factors, which come to dominate at elevated temperature.
Problem OC3.7.
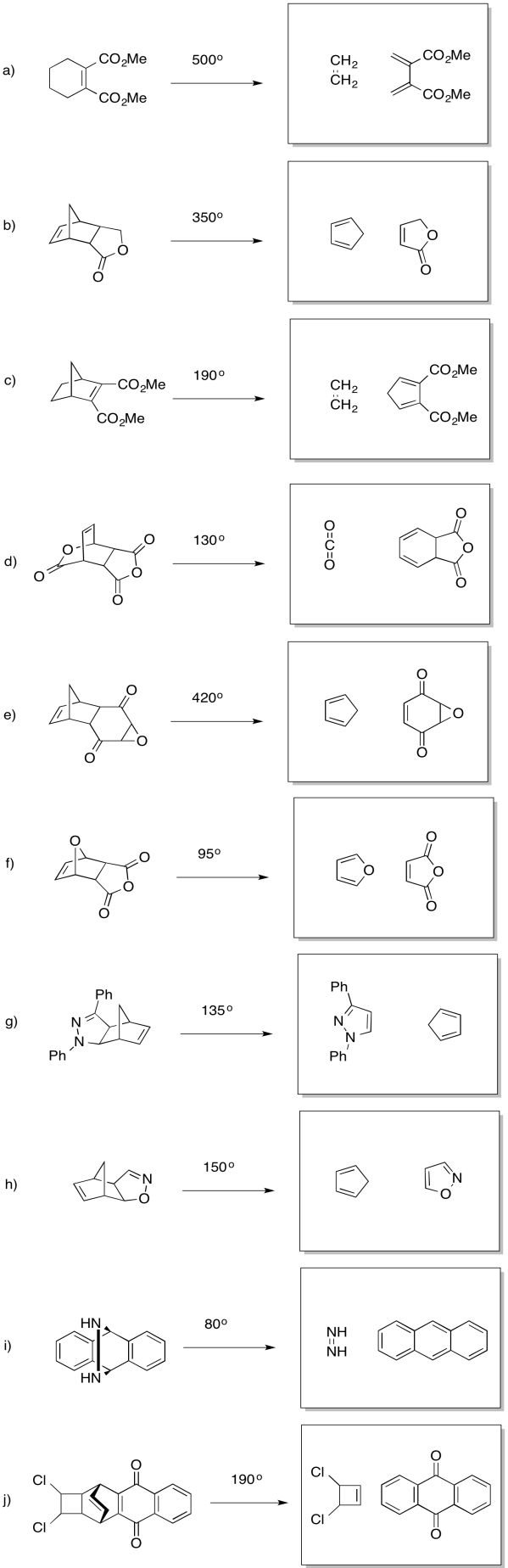
Problem OC3.8.
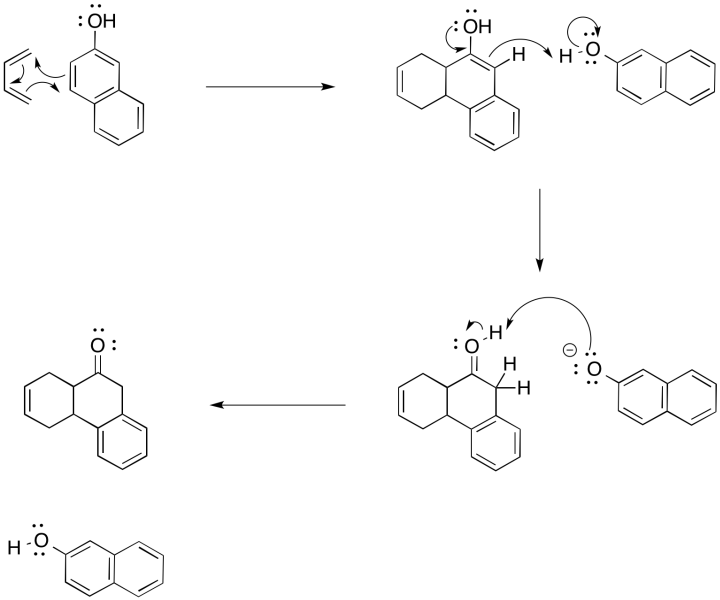
Problem OC4.1.
The maleic anhydride is polarized, with electron density drawn toward the anhydride functional group on one side of the ring. As a result, the other side of the ring is left more positive, and will attract the polarizable electron density from the diene.
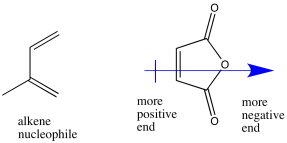
Problem OC4.2.
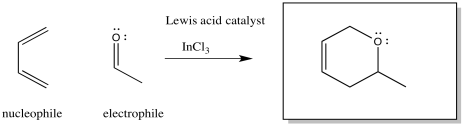
Lewis acids frequently activate carbonyl compounds towards interaction with nucleophiles. They do so by binding to the lone pairs on the carbonyl oxygen, drawing electron density away from the oxygen and, inductively, away from the neighbouring portion of the molecule.
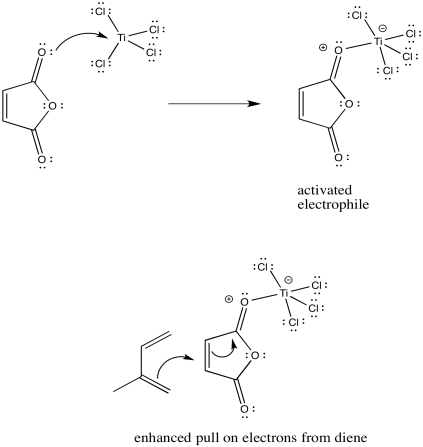
Problem OC4.3.
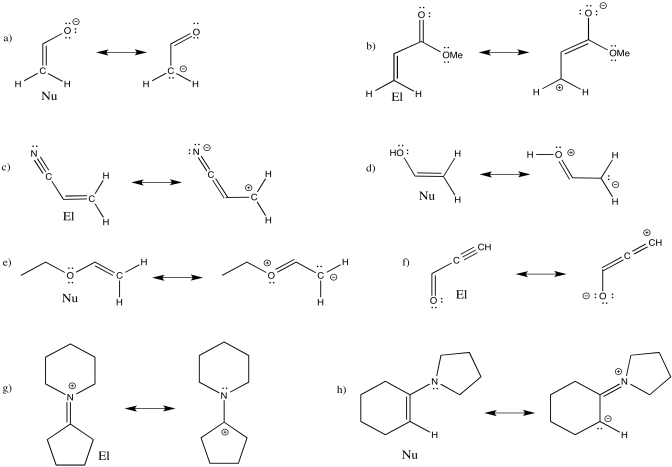
Problem OC4.4.

Problem OC4.5.

Problem OC4.6.
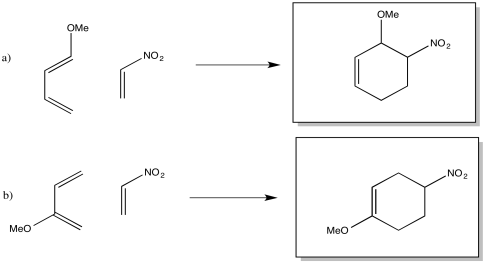
Problem OC4.7.
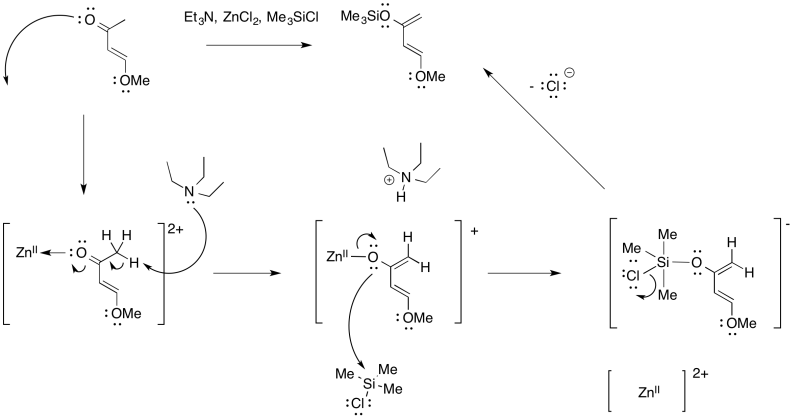
Problem OC4.8.
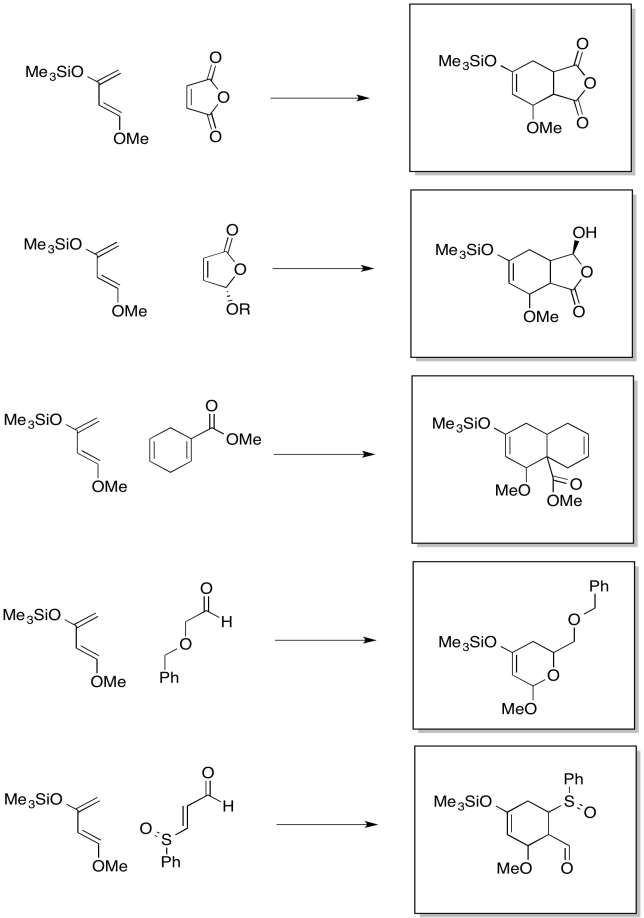
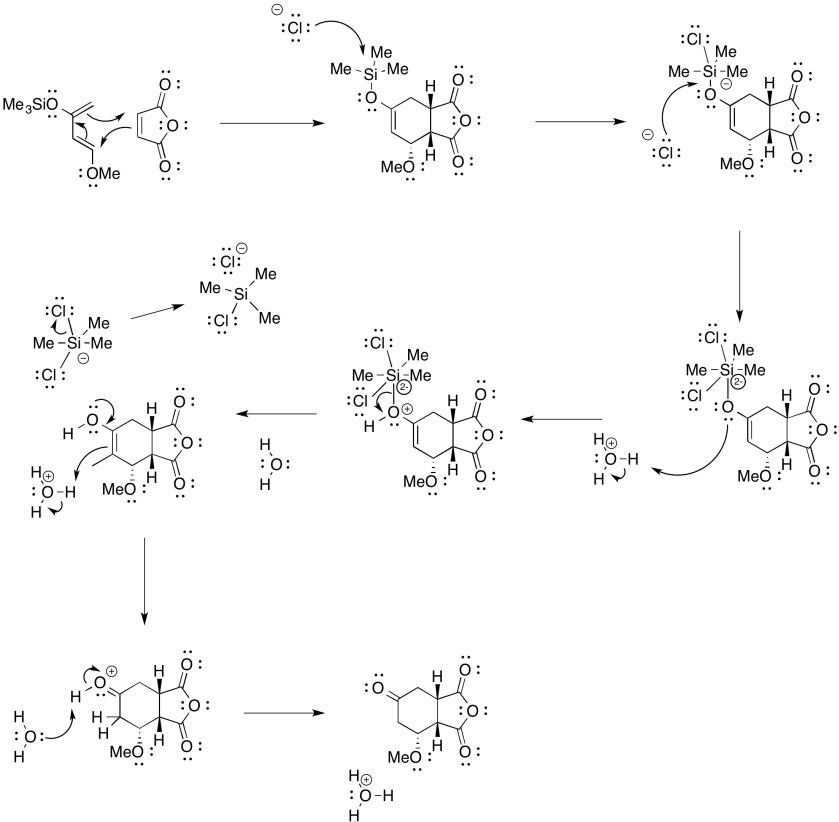
Problem OC4.9.
Problem OC4.10.
Problem OC4.11.
Problem OC4.12.
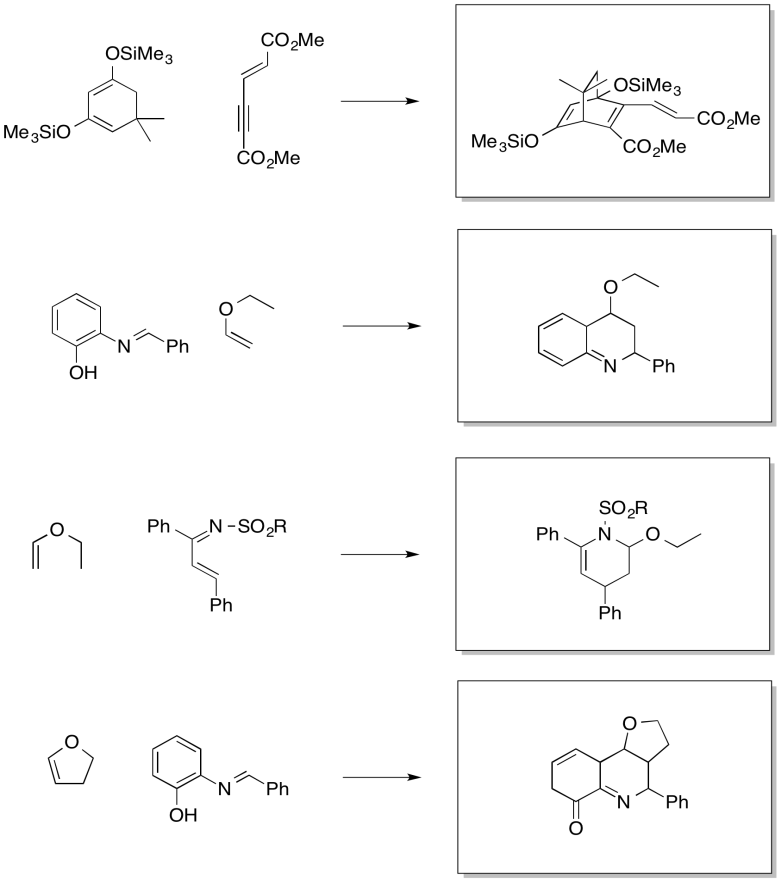
Problem OC4.13.
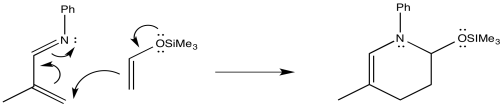
The drawings indicate the two electron-donating groups are operating at cross purposes. One directs electron density toward one end of the diene, whereas the other directs the electron desnity toward the other end.

Problem OC5.1.
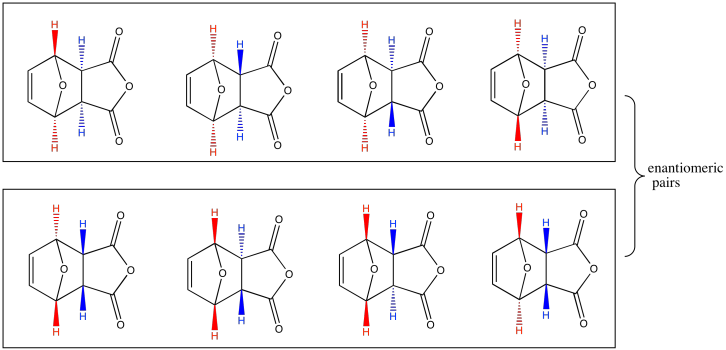
Problem PR5.2.
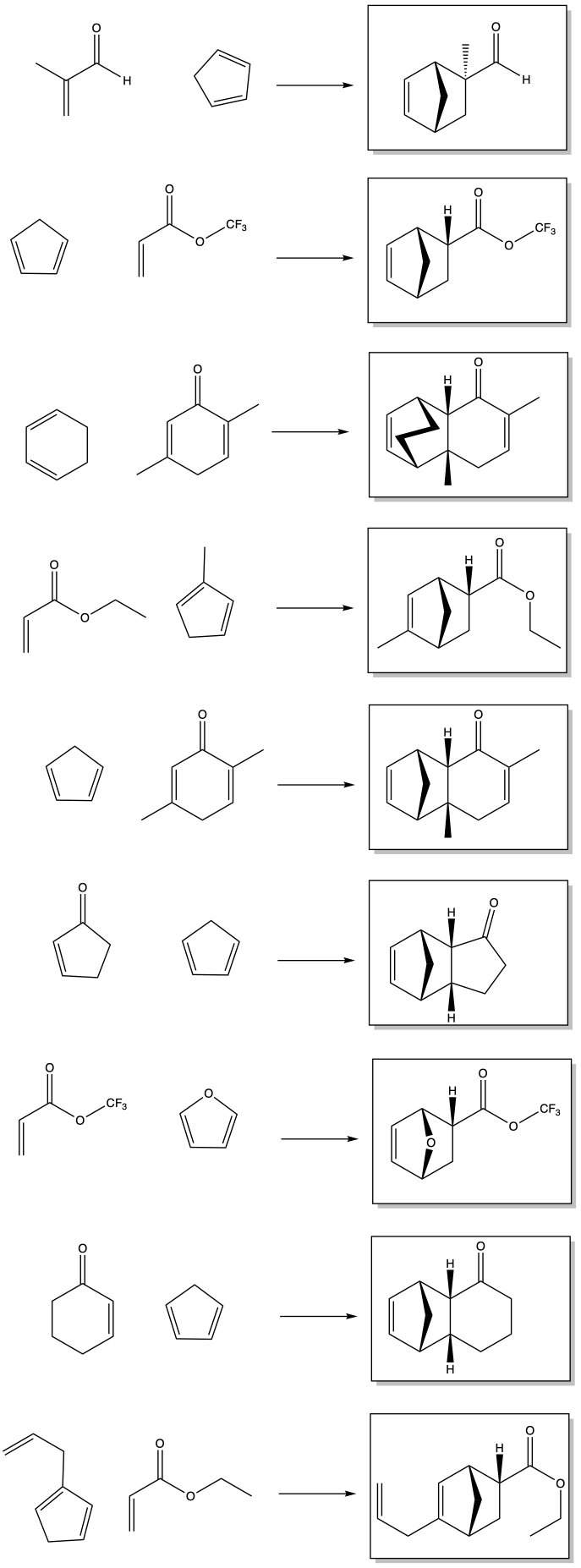
Problem OC8.1.
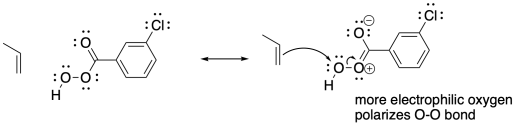
Problem OC8.2.
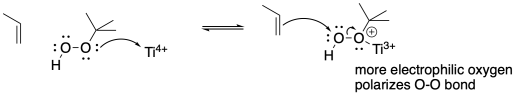
Problem OC8.3.
a) OsO4, H2O
b) mCPBA
c) 1. O3; 2. Me2S or Zn
d) 1. O3; 2. Me2S or Zn
e) OsO4, H2O
f) mCPBA
Problem OC8.4.
Problem OC8.5.
Problem OC8.6.
Problem OC8.7.
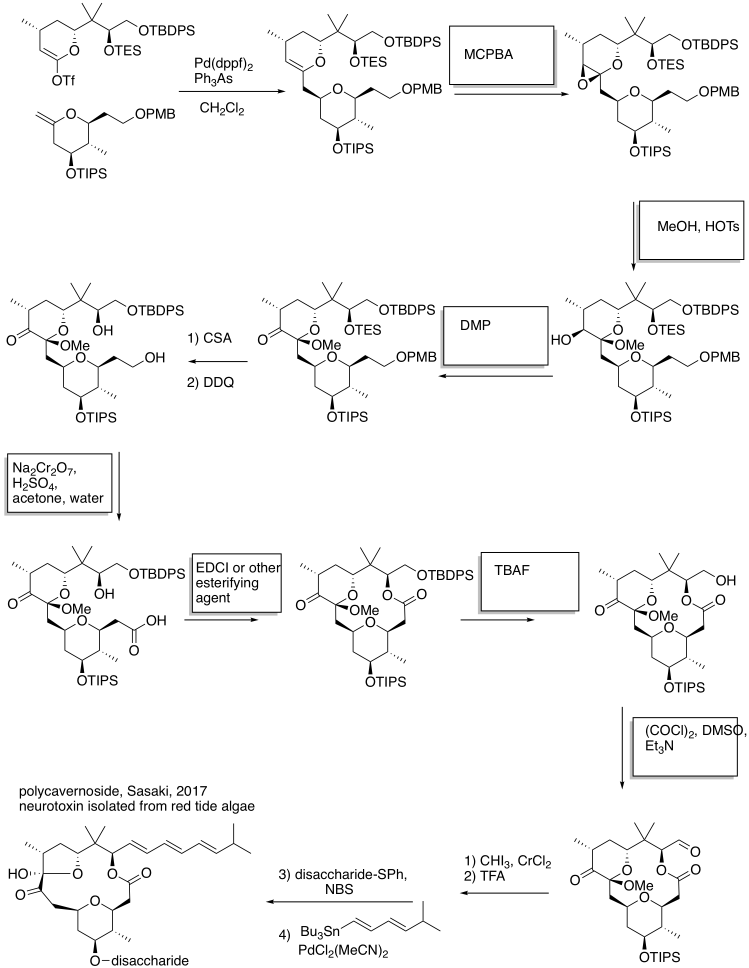
Problem OC9.1.
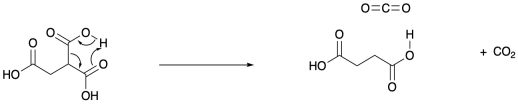
Problem OC9.2.
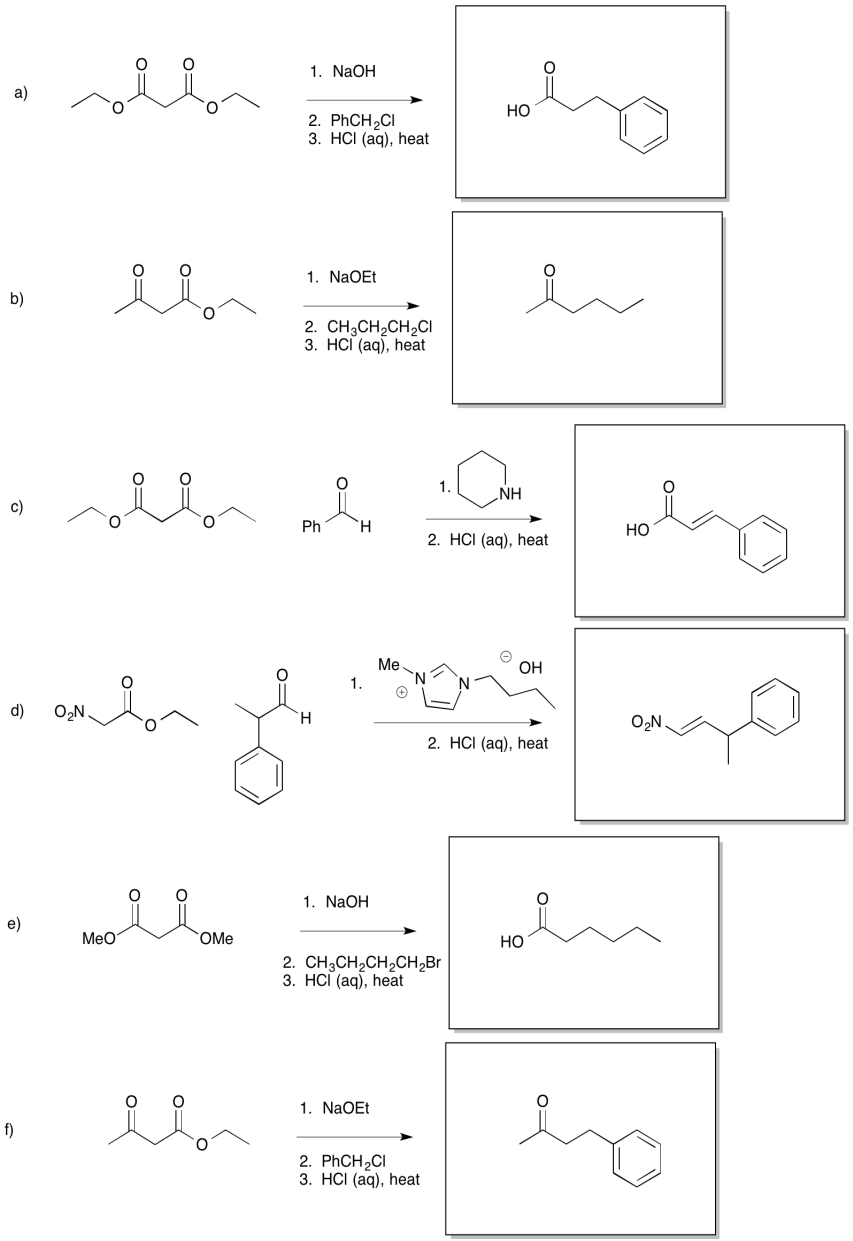
Problem OC9.3.
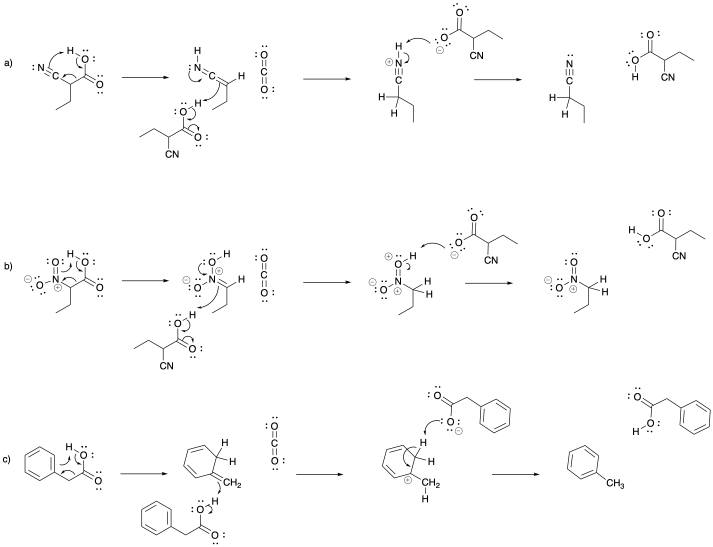
Problem OC9.4.
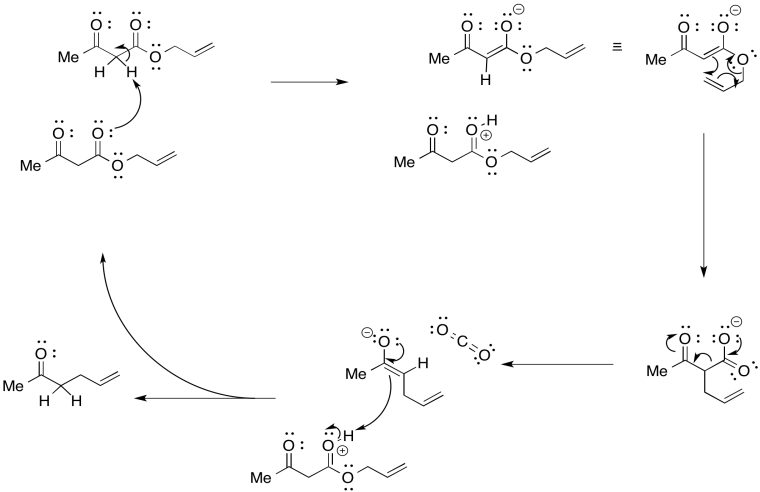
Problem OC10.1.
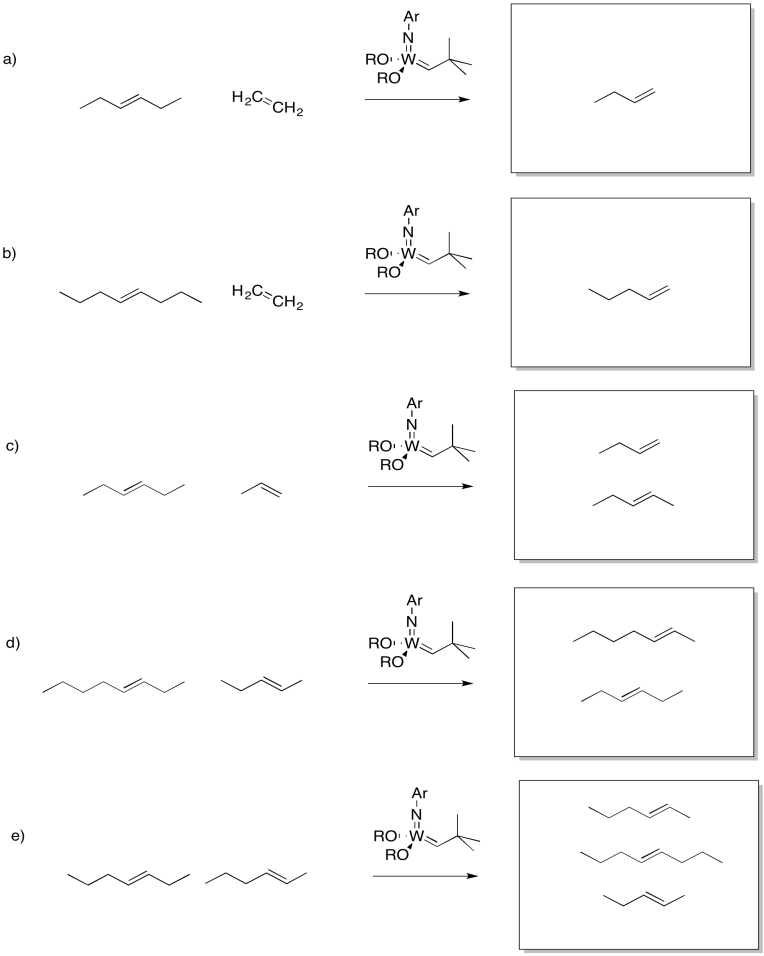
Problem OC10.2.
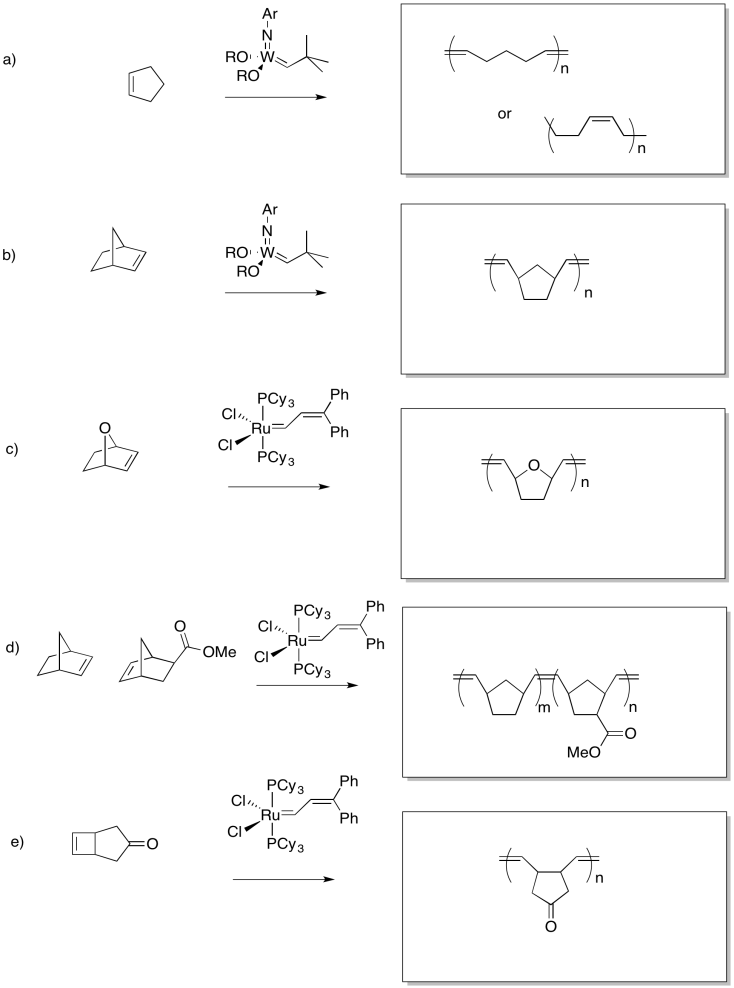
Problem OC10.3.
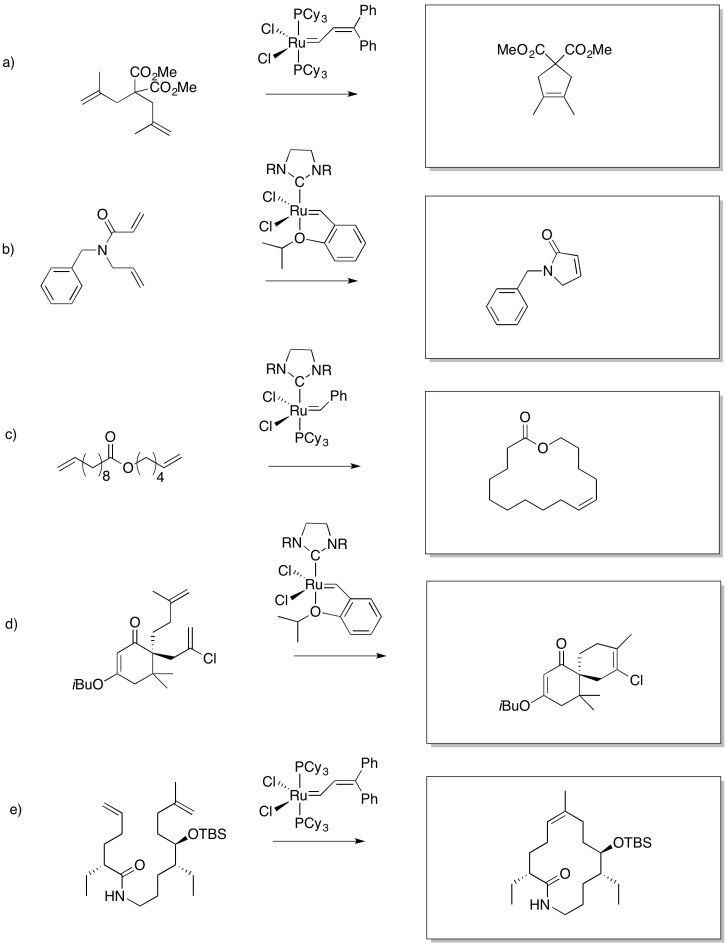
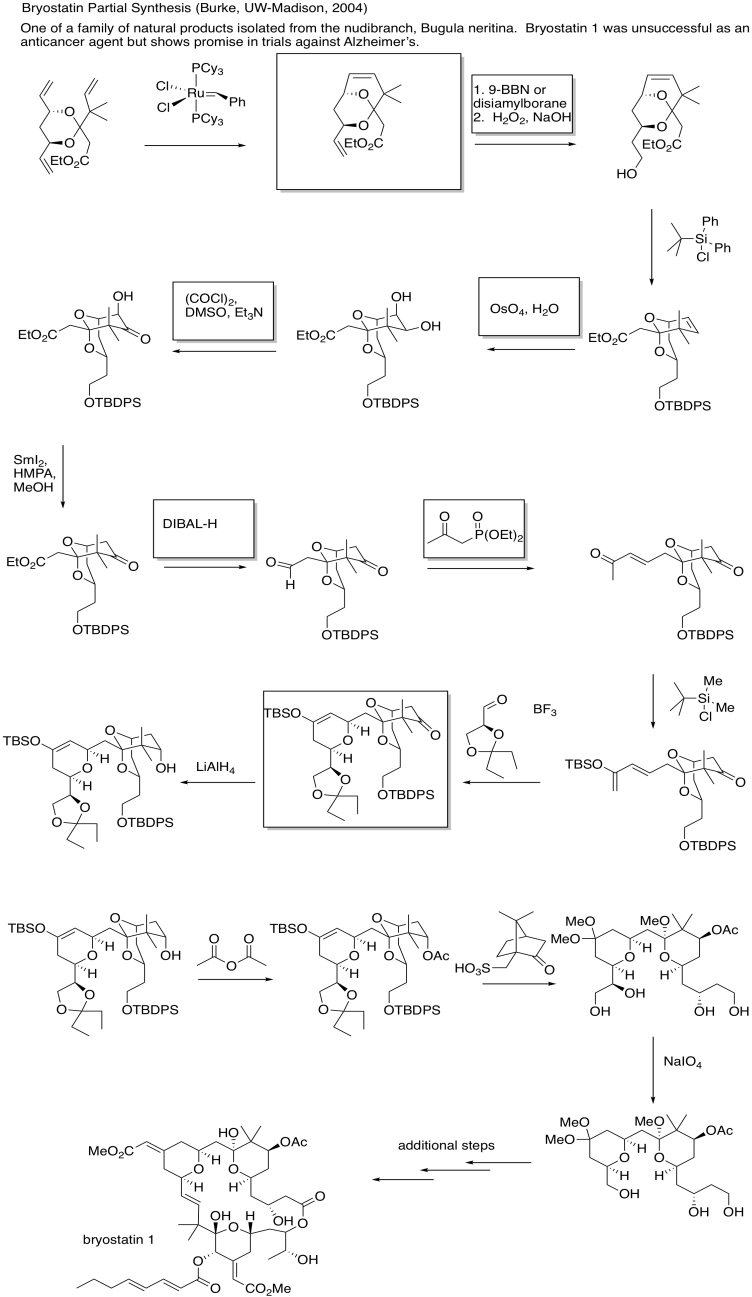
Problem OC10.4.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: