Reactivity in Chemistry
Radical Reactions
RR3. Radical Initiation: Radical Stability
Bond strength isn't just about the interaction of the two fragments bonded together. It is also influenced by the stability of those two species on their own. When the bond is broken, what pieces are left over?
The formation of radicals may be driven by the weakness of a particular bond. In terms of radical formation via bond homolysis, the reaction is more product-favoured if the bond being broken is weak. In other words, the bond is not very low in energy, so the overall reaction may become more downhill (or at least less uphill). In that case, forward reaction is favoured because of reactant destabilization.
However, a downhill reaction could also occur through product stabilization. For example, we have already seen that larger, more polarizable atoms form more stable radicals. Iodine radicals are more stable than bromine radicals, and sulfur radicals are more stable than oxygen radicals.
There are other factors, too. One of the most important factors is resonance. We have seen that the stability of anions and cations is strongly influenced by delocalization. Factors that spread the excess charge onto multiple atoms, rather than allowing charge to concentrate on one atom, make charged species much more stable.
For example, carbon-based anions are relatively unstable, but a delocalized carbanion is within the realm of possibility. Enolate ions are particularly easy to obtain because negative charge is partially delocalized onto a more electronegative oxygen atom. Delocalization also strongly stabilizes radicals. It is one of the most important factors in the stability of carbon-based radicals.
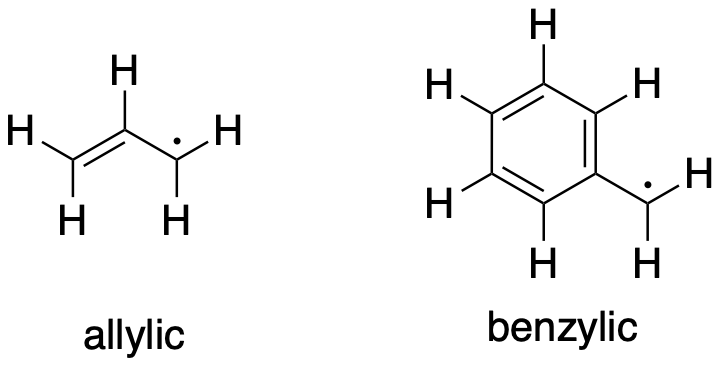
Figure RR3.1. Two very stable radicals.
Problem RR3.1.
Illustrate the resonance stabilization in the following radicals
a) allyl, CH2CHCH2 b) benzyl, CH2C6H5 c) cyclopentadienyl, C5H5
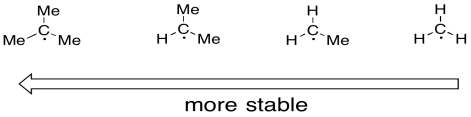
Radicals on carbon atoms are also stabilized when they are in more substituted positions. just as carbocations are more stable if they are on more substituted positions, carbon radicals are also more stable in these positions. A tertiary radical is more stable than a secondary one. A secondary radical is more stable than a primary one.

Figure RR3.2. Radical stability depends on the degree of substitution.
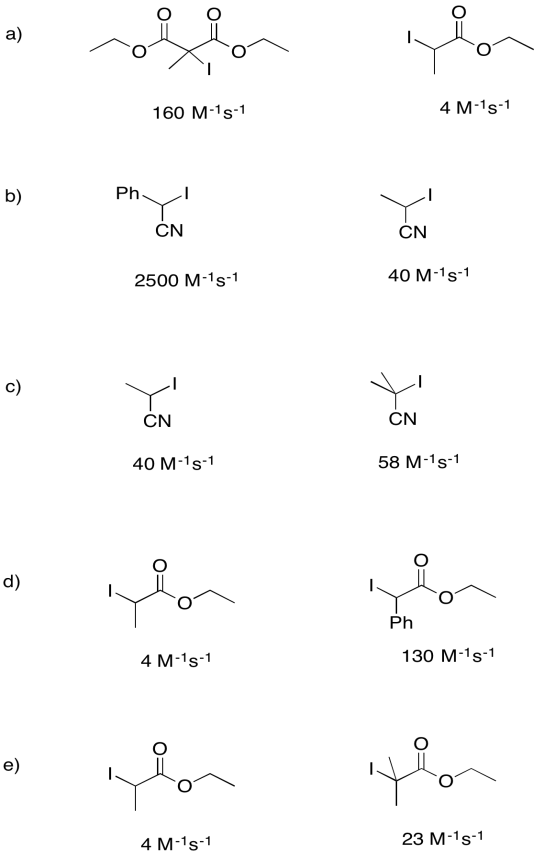
Problem RR3.2.
Rate constants for the dissociation of the following initiators to form an iodine atom and a radical were measured under a specific set of conditions. For each pair, explain why one compound undergoes homolysis more quickly.
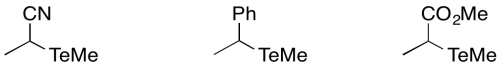
Problem RR3.3.
Predict the relative order of bond dissociation rates for the following initiators, which would each form a tellerium radical and a carbon radical.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: