Reactivity in Chemistry
Electrophilic Rearrangement
ER3. Baeyer-Villiger Rearrangement
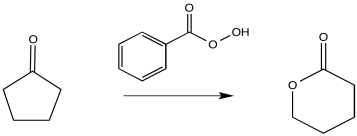
The Baeyer-Villiger rearrangement is the conversion of a ketone to an ester via the insertion of an oxygen atom next to the carbonyl.

Ficture ER3.1. A Baeyer-Villiger rearrangement.
The reaction involves initial addition of a peroxide to the carbonyl carbon. A peroxide is a compound that contains an extra oxygen, essentially. In general, peroxides contain weak O-O single bonds. That bond is easily broken and an oxygen atom is donated to another compound. The resulting adduct undergoes rearrangement to form the ester.
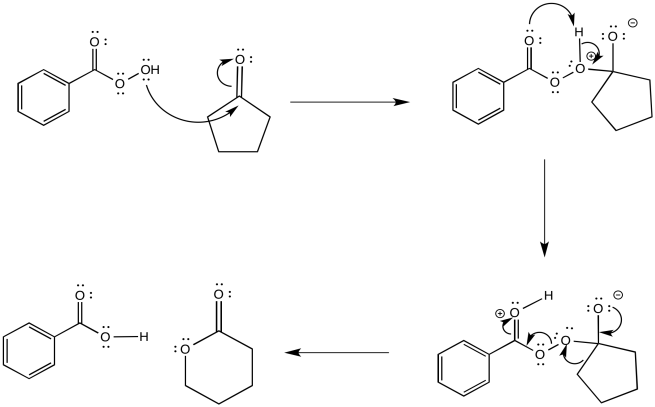
Figure ER3.2. The mechanism of the Baeyer-Villiger features a 1,2-shift of the electrons in a C-C bond to become a C-O bond.
The reagents for Baeyer-Villiger oxidation include the perbenzoic acid shown above, as well as several others. Probably the most common reagent historically is meta-chloroperbenzoic acid or m-CPBA. This compound is very efficient for this reaction but it does pose some safety issues, especially during shipping, and it leaves behind a chlorinated by-product. In general, halogenated waste compounds pose environmental hazards. Magnesium monoperoxyperephthalate (MMPP) was developed as a safer alternative. However, it still produces an appreciable amount of organic by-product. Even greener reagents include hydrogen peroxide. By itself, the by-product of oxygen donation would be water, but hydrogen peroxide isn't very good at the Baeyer-Villiger reaction so Lewis acids, such a boron trifluoride, must be added to activate the reagent. Another very common reagent is oxone, a commercially available mixture of 2:1:1 potassium persulfate:potassium bisulfate:potassium sulfate, Oxone is also consider a green reagent because the sulfate by-products are relatively safe. These by-products are also water-soluble, so they are really easy to remove from the organic product.
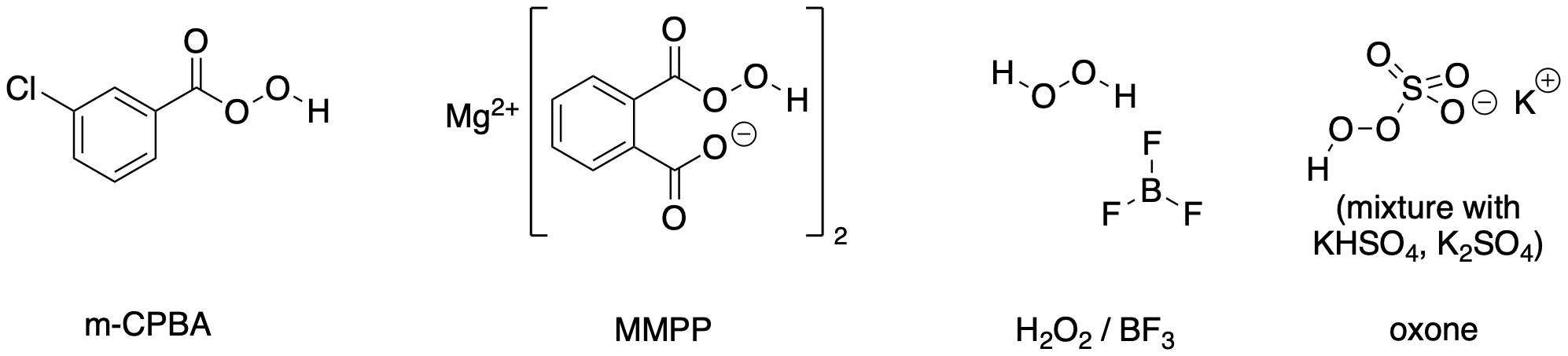
Figure ER3.3. Some reagents used for Baeyer-Villiger oxidations.
The Baeyer-Villiger reaction does show some regioselectivity. If the starting ketone is not symmetric, the oxygen atom can be a inserted preferentially into one side of the ketone rather than the other. This preferential insertion generally happens when the carbon directly attached on one side of the ketone is more highly substituted than the carbon on the other side. For example, if one carbon is tertiary and the other carbon is secondary, then the oxygen atom inserts between the tertiary carbon and the carbonyl.
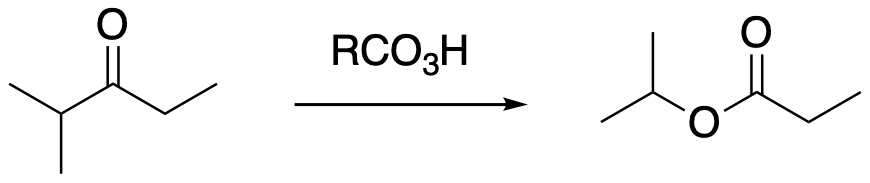
FigureER3.4 The Baeyer-Villiger oxidation leads to the new C-O bond forming with the most substituted carbon.
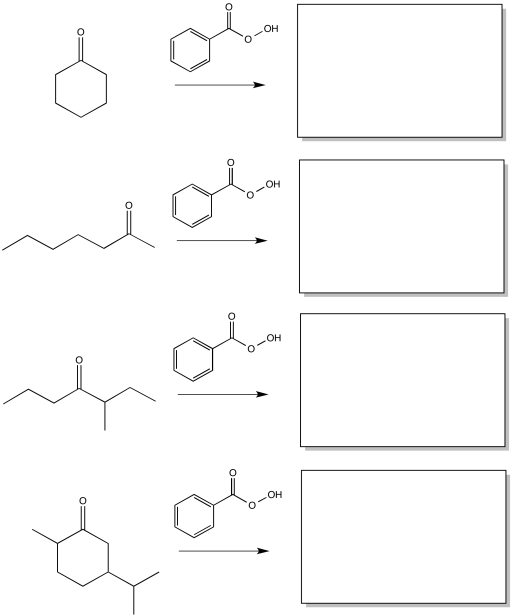
Problem ER2.1.
Predict the products of the following Baeyer-Villiger reactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: