Reactivity in Chemistry
Reduction & Oxidation Reactions
RO10. Inner Sphere Electron Transfer
In some cases, electron transfers occur much more quickly in the presence of certain ligands. For example, compare the rate constants for the following two electron transfer reactions, involving almost exactly the same complexes:
Co(NH3)63+ + Cr2+ → Co2+ + Cr3+ + 6 NH3 k = 10-4 M-1 s-1
Co(NH3)5Cl2+ + Cr2+ → Co2+ + CrCl2+ + 6 NH3 k = 6 x 105 M-1 s-1
(Note: aqua ligands are omitted for simplicity. Ions, unless noted otherwise, are aqua complexes.)
Notice two things: first, when there is a chloride ligand involved, the reaction is much faster. Second, after the reaction, the chloride ligand has been transferred to the chromium ion. Possibly, those two events are part of the same phenomenon.
Similar rate enhancements have been reported for reactions in which other halide ligands are involved in the coordination sphere of one of the metals.
In the 1960's, Henry Taube of Stanford University proposed that halides (and other ligands) may promote electron transfer via bridging effects. What he meant was that the chloride ion could use one of its additional lone pairs to bind to the chromium ion. It would then be bound to both metals at the same time, forming a bridge between them. Perhaps the chloride could act as a conduit for electron transfer. The chloride might then remain attached to the chromium, to which it had already formed a bond, leaving the cobalt behind.
Electron transfers that occur via ligands shared by the two metals undergoing oxidation and reduction are termed "inner sphere" electron transfers. Taube was awarded the Nobel Prize in chemistry in 1983; the award was based on his work on the mechanism of electron transfer reactions.
Problem RO10.1.
Take another look at the two electron transfer reactions involving the cobalt and chromium ion, above.
a) What geometry is adopted by these complexes?
b) Are these species high spin or low spin?
c) Draw d orbital splitting diagrams for each complex.
d) Explain why electron transfer is accompanied by loss of the ammonia ligands from the cobalt complex.
e) The chloride is lost from the cobalt comples after electron transfer. Why does it remain on the chromium?
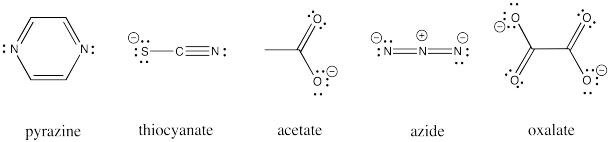
Other ligands can be involved in inner sphere electron transfers. These ligands include carboxylates, oxalate, azide, thiocyanate, and pyrazine ligands. All of these ligands have additional lone pairs with which to bind a second metal ion.

Figure RO10.1. Some examples of bridging ligands other than halides
Problem RO10.2.
Draw an example of each of the ligands listed above bridging between a cobalt(III) and chromium(II) aqua complex.
Problem RO10.3
Explain, with structures and d orbital splitting
diagrams, how the products are formed in the following reaction, in aqueous
solution.
Fe(OH2)62+ + (SCN)Co(NH3)52+
→ (NCS)Fe(OH2)52+ + Co(OH2)62+
+ 5 NH3
How does the electron travel over the bridge?
Once the bridge is in place, the electron transfer may take place via either of two mechanisms. Suppose the bridging ligand is a chloride. The first step might actually involve an electron transfer from chlorine to the metal; that is, the chloride could donate one electron from one of its idle lone pairs. This electron could subsequently be replaced by an electron transfer from metal to chlorine.
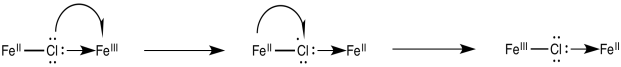
Figure RO10.2. An example of a bridging ligand carrying a "hole".
Sometimes, we talk about the place where an electron used to be, describing it as a "hole". In this mechanism, the electron donated from the bridging chloride ligand leaves behind a hole. The hole is then filled with an electron donated from the other metal.
Alternatively, an electron might first be transferred from metal to chlorine, which subsequently passes an electron along to the other metal. In the case of chlorine, this idea may seem unsatisfactory, because chlorine already has a full octet. Nevertheless, some of the other bridging ligands may have low-lying unoccupied molecular orbitals that could be populated by this extra electron, temporarily.
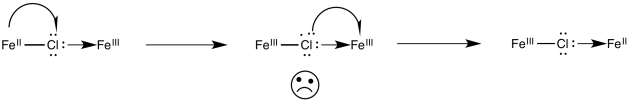
Figure RO10.3. An example of a bridging ligand carrying an extra electron.
Thiocyanate, for example, has a low-lying π* orbital that could accommodate an extra electron. This mechanism could work with SCN- or other brindging ligands with π bonds.

Figure RO10.4. An example of a bridging ligand carrying an extra electron using a π* orbital.
Inner sphere electron transfers are not limited to transition metal complexes. In organic compounds, inner sphere transfer is possible when the donor and the acceptor are able get close enough together that donor and acceptor orbitals can overlap. This phenomenon might be seen in two aromatic comounds, for example. The planar rings can easily stack on top of each other and can be held together by London dispersion forces. That arrangement allows an electron to transfer directly from one compound to the other, rather than having to travel through some medium between the compounds.
Problem RO10.4.
For the iron / cobalt electron transfer in problem RO9.3., show
a) an electron transfer mechanism via a hole migration along the bridge
b) an electron transfer mechanism via an electron migration along the bridge
Problem RO10.5.
One of the many contributions to the barrier for electron transfer between metal ions is internal electronic reorganization.
a)
Draw d orbital splitting diagrams for each of the following metal ions in an
octahedral environment.
Ru(II) or Os(II)
Ru(III) or Os(III)
Co(II)
Co(III)
Flash photolysis is a method in which an electron can be moved instantly �uphill� from one metal to another (e.g. from M2II to M1III, below); the electron transfer rate can then be measured as the electron �drops� back from M1II to M2III.
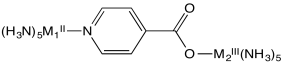
b) Explain the relative rates of electron transfer reaction in this system, as measured by flash photolysis in the table below.
| M1II | M2III | kobs s-1 |
| Os | Ru | > 5 x 109 |
| Os | Co | 1.9 x 105 |
c) Does the reaction above probably occur via an inner sphere or by an outer sphere pathway? Why?
Problem RO10.6.
Outer sphere electron transfer rates depend on the free energy change of the reaction (ΔG°) and the distance between oxidant and reductant (d) according to the relation
Rate constant = k = Ae(-ΔG)e-d
a) What happens to the rate of the reaction as distance increases between reactants?
One potential problem in measuring rates of intramolecular electron transfer (i.e. within a molecule) is competition from intermolecular electron transfer (between molecules).
b) What would you do in the flash photolysis experiment above to discourage intermolecular electron transfer?
c) How could you confirm whether you were successful in discouraging intermolecular reaction?
Problem RO10.7.
Stephan Isied and coworkers at Rutgers measured the following electron transfer rates between metal centers separated by a peptide. (Chem Rev 1992, 92, 381-394)
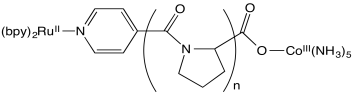
a) The proline repeating unit is crucial in ensuring a steady increase in distance between metal centers with increased repeat units, n. Why?
b) An inner sphere pathway in this case is expected to be somewhat slow because of the lack of conjugation in the polyproline bridge. Explain why.
c) Plot the data below, with logk on the y axis (range from 4-9) and d on the x axis (12-24 Angstroms).
| n | d (Å) | kobs (s-1 ) |
| 1 | 12.2 | 5 x 108 |
| 2 | 14.8 | 1.6 x 107 |
| 3 | 18.1 | 2.3 x 105 |
| 4 | 21.3 | 5.1 x 104 |
| 5 | 24.1 | 1.8 x 104 |
d) A linear relationship is in agreement with Marcus
theory; logk = - c x d. Is your plot linear?
Isied offers a number of possible explanations for the data, all of which involve two competing reaction pathways.
e) Suggest one explanation for the data.
Problem RO10.8.
Researchers at the University of Leeds reported a study of electron transfer between transition metal complexes (Geoffrey Thompson & Geoffrey Sykes, Inorg. Chem. 1976, 15 (3), 638-642.
The study involved two different reductants, [Ru(NH3)6]2+ and [Ti(OH2)6]3+, and a series of related oxidants, such [Co(NH3)5X]2+ (X = anionic ligand) and [Co(NH3)5Y]3+ (X = neutral ligand).
a) Draw ligand field splitting diagrams for each of the three metals in these compounds.
b) State whether each compound is labile or inert and indicate the reason for your choice.
c) First consider electron transfer between the ruthenium complex and the cobalt complexes. Are these reactions more likely to occur via an inner sphere or an outer sphere mechanism? Why?
d) The researchers measured rates of electron transfer between the ruthenium and each of the cobalt complexes.
The researchers also looked at electron transfer between the titanium complex and the cobalt complexes.
They made a plot of log kTi vs log kRu; if linear, such a plot indicates the two reactions go through the same mechanism.
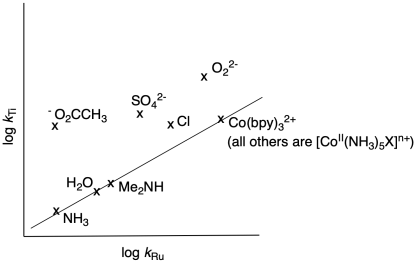
Some of the compounds fall along a line. What mechanism do they undergo?
e) Some of the compounds do not fall along the line. Are they faster or slower than expected?
f) What mechanism operates for this last group of compounds?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: