 Vaccines: Victims of Their Own Success?
Vaccines: Victims of Their Own Success?
Why the most effective public health intervention evokes a mixed response from the public | By Ricki Lewis
 |
|
|
Perhaps in no area is the divide between the developed and developing worlds as striking as it is for vaccines: While healthcare consumers in economically advantaged nations worry about risk, in developing nations compelling need forces a focus on potential benefit. "People in the United States want a quick solution, not prevention, so they prefer drugs to vaccines. Elsewhere, people are afraid of drugs and side effects, and prefer vaccines," says Shan Lu, a primary-care physician who has worked in many countries. He is developing an HIV vaccine at the University of Massachusetts, Amherst, where he is associate professor of infectious diseases and immunology.
In 2002, more than 2 million people died worldwide from vaccine-preventable illness, according to the World Health Organization (WHO) and the Geneva-based Global Alliance for Vaccines and Immunization (GAVI). While some healthcare consumers in privileged nations eschew vaccines because of side effects, real or perceived, elsewhere limited access to vaccines translates into rampant disease and death. Adding to the imbalance is that the same disease can have markedly different outcomes depending on the healthcare infrastructure of a nation. Constraints on vaccine use are complex and intertwined, involving sociology, economics, politics, science, and technology.
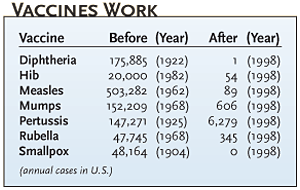
THE ROLE OF RISK Some antivaccine sentiment stems, ironically, from success. "In developed countries, we no longer have infectious diseases for which there are vaccines, so the risk of the vaccine is perceived to be greater than the risk of the disease. But that is true because the vaccine is being used," says Stanley Plotkin, professor emeritus at the University of Pennsylvania, science advisor to vaccine manufacturer Aventis-Pasteur, and inventor of the rubella vaccine.
Polio dramatically illustrates vaccine complacency. "In the 1950s, polio affected every neighborhood. Now natural polio is far removed from most peoples' daily lives. But when a little girl in California develops side effects from polio vaccine, that hits the newspapers," says Neil Herendeen, director of pediatrics at Strong Memorial Hospital at the University of Rochester, New York. Also, parents are more likely to question the necessity of vaccines today. "In the past, medicine was provided under a more paternalistic model, with the public trusting that they were receiving the appropriate service. Today, people participate more in their care, know more, and expect more," says Mark Upfal, corporate medical director at Detroit Medical Center Occupational Health Services.
 |
|
|
Rotavirus infection exemplifies how location can drastically affect disease outcome, and therefore need for a vaccine. "Rotavirus is the leading cause of diarrhea in infants and young children worldwide, with equal probability across socioeconomic and geographic groups. Nearly all children have it by age 5," says Penny Heaton, director of biologics clinical research at Merck Research Laboratories in West Point, Pa. Each year worldwide, rotavirus makes 111 million children mildly ill, 25 million sick enough to require a clinic visit, sends 2 million to the hospital, and kills 440,000,1 and the poorest countries bear 82 percent of the mortality burden. "In the US, people with rotavirus infection get sick, go to the hospital, and although it costs a lot for care, they don't die. Across the world where there are no intravenous fluids or oral rehydration or doctors to intervene, people die," explains Herendeen.
So when Wyeth Pharmaceuticals, then in Marietta, Pa., offered a rotavirus vaccine in 1998, the global impact was "enormous," Herendeen recalls. But nine months after routine vaccination began in children less than 2 years of age in the United States, a side effect called intussusception (a type of bowel obstruction) appeared in 1 in 12,000 vaccinees. When case-controlled studies by the Centers for Disease Control and Prevention (CDC) confirmed an association between the vaccine and intussusception onset, although not establishing cause, Wyeth withdrew the vaccine everywhere. Several new rotavirus vaccines are now in clinical trials.
VACCINOPHOBIA People avoid vaccines for several reasons. The paramount reason, fear, knows no geographic or cultural boundaries. Consider the resurgence of polio in the Nigerian states of Kano and Zamfara, following a boycott of the WHO's Global Polio Eradication Initiative. "Of the 800 cases of polio reported in 2003, half were in Nigeria, where there are rumors that the vaccine causes female infertility. And they have exported polio to nine neighboring countries," says David Heymann, head of the WHO campaign. Boycotters feared that the US government had added contraceptives to the vaccine to control population growth. But the Organization of the Islamic Conference, representing 56 nations, has endorsed polio vaccination, and Nigeria and 10 neighbors will resume their vaccination programs.
In the United Kingdom, decreasing rates of measles-mumps-rubella (MMR) vaccination have paralleled reappearance of the diseases. Parents have shunned "the jab" out of fear that the vaccine causes autism, a still-contentious link based on many anecdotal reports and a few studies. Yet some investigations seem open to interpretation. An early one, for example, investigated autistic-like behavior in a dozen children with a rare gastrointestinal disorder whose behavioral changes ensued after MMR vaccination.2 But the children were possibly not representative of others with autism, and most of the authors recently retracted their speculations.3 Several much larger, controlled studies did not find a link, but Congressman Dave Weldon (R-Fla.), a physician, sent a letter to CDC director Julie Gerberding in October 2003, criticizing one influential report for manipulating data to dampen vaccine-autism associations. He also cited a conflict of interest of researchers accepting funding from vaccine manufacturers.4
While the physicians argue, parent groups continue to pursue thimerosal as a cause of autism. Lobbyist Rick Rollens from Granite Bay, Calif., testified before Congress about his son, Russell. "Surely any intelligent, thoughtful person cannot with a straight face suggest that the huge increase in one of the most easily recognizable of all childhood disorders is all due to genetics, better recognition, or to minor changes in the diagnostic criteria that occurred 10 years after the massive increase in autism had already begun over two decades ago."
Considering the genetic background of autistic children may shed some light on causality. A study on mice at Columbia University supports the hypothesis that a genetic susceptibility might make some individuals more vulnerable to toxic effects of mercury.5 Associate professor of epidemiology Mady Hornig and colleagues exposed mice whose immune system genes were associated with sensitivity to the preservative, delivered either alone or in four common vaccines given to children, and control mice. Behavioral and brain changes in animals given thimerosal in either form resembled autism. The researchers based their experimental design on the observation that some children with autism have a family history of autoimmune disease.
However, a report from the Institute of Medicine, Washington, DC, in May 2004 concludes that this and several other studies do not establish a causative connection between thimerosal exposure and autism. Committee chair Marie McCormick, a Sumner and Esther Feldberg Professor of Maternal and Child Health at the Harvard School of Public Health, said, "We strongly support ongoing research to discover the cause or causes of this devastating disorder. Resources would be used most effectively if they were directed toward those avenues of inquiry that offer the greatest promise for answers. Without supporting evidence, the vaccine hypothesis does not hold such promise."
Dismissing entire areas of research, however, may be counterproductive, says Hornig. "We believe that these conclusions may have been rendered prematurely. Our findings of a possibly restricted genetic susceptibility to the effects of low-dose thimerosal in vaccines suggests that the design of published epidemiologic studies may have been inadequate to appropriately estimate risk."
People are more likely to accept the small risk of a vaccine if the disease it prevents is familiar and sufficiently dire or disruptive. Consider a different set of vaccines:
- In February 2002, SmithKline Beecham (now GlaxoSmithKline) withdrew an effective Lyme disease vaccine from the market owing to poor sales. "Anecdotal reports of adverse events were widely circulated. There was a theoretical concern that the vaccine could cause autoimmune arthritis, but this was not supported by data," says Joseph Piesman, chief of entomology and ecology activity, bacterial zoonoses branch, at the CDC. Other factors that may have doomed the Lyme vaccine: The multidose protocol, effectiveness of antibiotics, and ease of avoiding ticks.
- Many first responders and medical personnel have avoided smallpox vaccination in the wake of the anthrax mail scare of 2001, because of serious side effects: In 2003, heart problems developed in 22 of 38,257 vaccinated civilians and 63 of 515,000 military personnel, and three people died. When similar side effects appeared in a Phase III trial of a new vaccine in April 2004, Acambis in Cambridge, UK, ceased recruitment. Several companies are developing a milder version, a modified vaccinia Ankara.
- Success of the chickenpox vaccine highlights the different mindset in the developed and developing worlds. The vaccine has a very low incidence of side effects and treats a usually mild disease. "What sells it most, though, is that if your child has chickenpox, you're home for a week. Can you afford to miss a week of work?" asks Herendeen.
- Despite the success of childhood vaccines, some parents question the crowded vaccine schedule for the toddler set, typically 18 injections in the US. But the human immune system can handle many more than the 100 or so antigens that the shots deliver.
Sharon Humiston, assistant professor of emergency medicine at the University of Rochester's Strong Memorial Hospital, offers the perspective of a pediatrician and parent of a child with autism. "In one day, my son licked a handrail at an airport, kissed the dog on the mouth, and ate what was coming out of his nose. A child is exposed to thousands of antigens in a normal day, and that dwarfs the number in vaccines." Indeed, technology has sharply reduced the number of antigens that vaccines introduce; the field has come a long way from smearing smallpox exudate into broken skin (see Retractions, Recommendations, and Regulations (US) (PDF, 345K).
ECONOMICS Creating a vaccine is expensive. A Phase III clinical trial alone can take more than three years and cost $50-300 million.6 For a company to take the plunge, a safe and effective product and a large, continual market are critical. But that's a tall order.
"Mass immunization is only recommended if a vaccine is relatively safe, offers good protection of about 80 to 90 percent, and is for an infection that is common in a particular poulation, such as Haemophilus influenzae for the very young, pneumococcal vaccine for the very old, and typhoid for travelers. The infection must also frequently cause death, such as influenza in the elderly, or illness, such as hepatitis B," explains Anthony Way, a professor of preventive medicine at Texas Tech University Health Sciences Center in Lubbock. In the United States, the CDC's Advisory Committee on Immunization Practices (ACIP) sets vaccine policy. "The committee consists of all major organizations and interest groups that have a stake in immunization policy," explains Steve Cochi, director of the CDC's national immunization program.
|
|
| ||
|
|
| ||
|
| |||
|
| |||
But what works on the economic balance sheet can pull at the heartstrings in real life. In the United States an adolescent or young adult's risk of contracting Niesseria meningitidis meningococcal infection is 1 in 125,000, and the risk of dying or enduring severe effects such as hearing loss, seizures, or gangrene is 1 in 1,250,000; these statistics are of little solace to loved ones of a patient who dies. The rarity of the condition, the high cost of the vaccine (about $80), and the short duration of protection (three years) provided by the current polysaccharide vaccine, has prompted ACIP to advise healthcare providers to counsel college freshmen living in dorms about the vaccine, rather than outright recommending it, hardly a ringing enough endorsement to encourage insurance coverage. The reason for ACIP's stance, says Way, "is that vaccinating all dormitory freshmen every year would only prevent one to three deaths at a cost of $7 million to $20 million per life saved." However, recommendation may come with anticipated Food and Drug Administration approval of a longer-lasting conjugate vaccine in early 2005, says Cochi.
Epidemiology is also part of the economic equation. Prevalence and/or spread of a disease must be significant, which is why West Nile virus (WNV) infection quickly captured the attention of vaccine developers. "We noticed the emergence of this disease in 1999. As a flavivirus related to another vaccine we had in development, for Japanese encephalitis, it was an attractive target for at least exploratory research," relates Philip Bedford, senior vice president of clinical operations and regulatory affairs at Acambis. WNV infection has exploded from 62 cases in 1999 to 9,858 in 2003.
Only 1 in 150 people infected with WNV become severely ill, with encephalitis developing with a 1 in 10 fatality rate. Nonetheless, the many infected individuals provide a sizeable market. Acambis' Phase I vaccine is a live attenuated yellow fever virus with WNV coat proteins.
A shot in the arm for vaccine research is the focus on bioterrorism. The Project BioShield Initiative passed Congress in May 2004, providing $5.6 billion for counterterrorism measures that include vaccine research, development, production, and distribution. The project will likely overreach its mandate, like technological spinoffs from the space program. "We are building the public health infrastructure to make vaccines rapidly and to recognize new pathogens as they emerge, such as SARS and pandemic flu," says John Treanor, associate professor of medicine at the University of Rochester, New York, who led recent smallpox vaccine trials.
Meningococcus is rare, WNV infection usually mild, and bioterrorism largely a theoretical threat. In contrast, in some developing nations, infectious disease is more immediately devastating. Consider a 2003 outbreak of Neisseria meningitides infection in Burkina Faso, West Africa, which killed 1500 and left hundreds with brain damage (the same pathogen that strikes dorm residents). WHO and other organizations had urged manufacturers and wealthy governments to provide vaccine in fall 2002 to prevent an epidemic; most preferred to wait until an outbreak occurred, a less costly option. The Bill and Melinda Gates Foundation provided most of the early aid.
The complexity of understanding disease processes and protection can also limit vaccine development, says Plotkin, citing the "big three" of HIV, tuberculosis (TB), and malaria. HIV's circumvention of vaccine efforts is legendary, and the live, weakened BCG (Bacillus Calmette-Guerin) vaccine against TB, available since 1922, is not highly effective. Malaria is particularly troublesome. "The parasite is complex, and it has many candidate antigens, possibly all working synergistically to produce immunity. We have to agree on which antigens to use in vaccines," Plotkin adds.
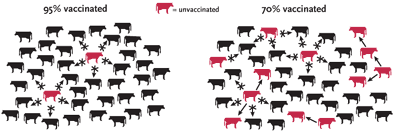
SPREADING THE GOOD WORD Vaccines save lives on a large scale, but only if enough individuals receive them to create "herd immunity." That is, if most members of a population are vaccinated, the few who aren't are unlikely to become infected, because the pathogen is not sustained. Herd immunity is possible only if people believe that the societal benefit of a vaccine is worth the risk the vaccine may pose.
 |
|
|
|
Because vaccine compliance is a sometimes-heated issue that requires education, groups and organizations are attempting to spread the word of vaccine efficacy, from the local level to the global:
- In Rochester, NY, social workers lead inner city residents to find children in need of vaccines, provided at Strong Memorial.
- Project Immunize Virginia is a statewide effort that promotes vaccination "across the lifespan," from kids in daycare to senior citizen centers, says coordinator Sara Nasca.
- The Basel, Switzerland-based Brighton Collaboration formed in February 2004 to standardize safety data to "provide consumers with a more harmonious and evidence-based picture of our knowledge of a given adverse event following immunization," says coordinator Katrin Kohl.
- WHO and UNICEF immunized more than 2 million children in Sudan in June 2004 against measles, a disease that is a killer in the face of malnutrition.
- GAVI is a public and private partnership whose "Campaign for Child Immunization" aims to save a million lives by 2006.
Globally, the vaccine challenge is daunting. "Twenty or more vaccines are needed, each one with a compelling public health need, but there are not enough ways to get vaccines into common use," sums up Plotkin. At the same time, in more fortunate nations some vaccines have suffered a tarnished reputation. But Bedford is hopeful that the image can change. "Vaccines do a lot more good than they seem to do harm. A small minority of people has waged a campaign against vaccines, and the resulting reemergence of diseases that we thought we'd eradicated is quite frightening. But if vaccines are seen to prevent disease, particularly if there is an unmet need or to replace a vaccine that is far from satisfactory, I think vaccine companies can gradually rebuild confidence for these very good preventive measures."
Ricki Lewis (ralewis@nycap.rr.com) is a freelance writer in Scotia, NY.
1. U.D. Parashar et al., "Global illness and deaths caused by rotavirus disease in children," Emerg Infect Dis, 9:565-72, 2003.
2. A.J. Wakefield et al., "Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children," Lancet, 351:637-41, 1998.
3. S.H. Murch et al., "Retraction of an interpretation," Lancet, 363:749, March 6, 2004
4. T. Verstraeten et al., "Safety of thimerosal-containing vaccine: a two-phased study of computerized health maintenance organization databases," Pediatrics, 112:1039-48, 2003.
5. M. Hornig et al., "Neurotoxic effects of postnatal thimerosal are mouse strain dependent," Mol Psychiatry, doi:10.1038/sj.mp.4001529, June 8, 2004.
6. G.J.V. Nossal, "A healthier climate for the funding of vaccine research," Nat Immunol, 5:457-9, May 2004.
|
| |||
|
|
| ||
|
| |||