WCB Quiz: Acid/Bases - Strength and Stability
Assume that each of the following reactants can react with liquid water to form H3O+ and the corresponding negatively charged products.
Products: CH3-, NH2-, HO-, and F-.
Answer questions 1-9 about these reactants and products.
1. Which product is most stable?
a. CH3-b. NH2-.
c. HO-
d. F-
2. Which product is most reactive?
a. CH3-b. NH2-.
c. HO-
d. F-
3. Which product is most basic?
a. CH3-b. NH2-.
c. HO-
d. F-
4. Which product is highest in energy?
a. CH3-b. NH2-.
c. HO-
d. F-
5. Which product is most likely to be formed?
a. CH3-b. NH2-.
c. HO-
d. F-
6. Which reactant is most acidic?
a. CH4b. NH3
c. H2O
d. HF
7. For which reactant is the reaction with water least favored?
a. CH4b. NH3
c. H2O
d. HF
8. For which reactant does the reaction with water have the most negative ΔGostab?
a. CH4b. NH3
c. H2O
d. HF
9. For which reactant does the reaction with water have the lowest pKa?
a. CH4b. NH3
c. H2O
d. HF
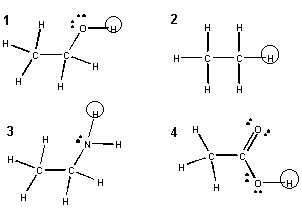
Assume that each of the following reactants can react with liquid water to form H3O+ and the corresponding negatively charged products. Assume the H's in circles are those protons which transfer to water.

Answer questions 10-18 about these reactants and products.
10. The product from which reactant is second to least stable?
a. 1b. 2
c. 3
d. 4
11. The product from which reactant is least reactive?
a. 1b. 2
c. 3
d. 4
12. The product from which reactant is least basic?
a. 1b. 2
c. 3
d. 4
13. The product from which reactant is second highest in energy?
a. 1b. 2
c. 3
d. 4
14. The product from which reactant is second most likely to be formed?
a. 1b. 2
c. 3
d. 4
15. Which reactant is second most acidic?
a. 1b. 2
c. 3
d. 4
16. For which reactant is the reaction with water lsecond least favored?
a. 1b. 2
c. 3
d. 4
17. For which reactant does the reaction with water have the most positive ΔGostab?
a. 1b. 2
c. 3
d. 4
18. For which reactant does the reaction with water have the second highest pKa?
a. 1b. 2
c. 3
d. 4