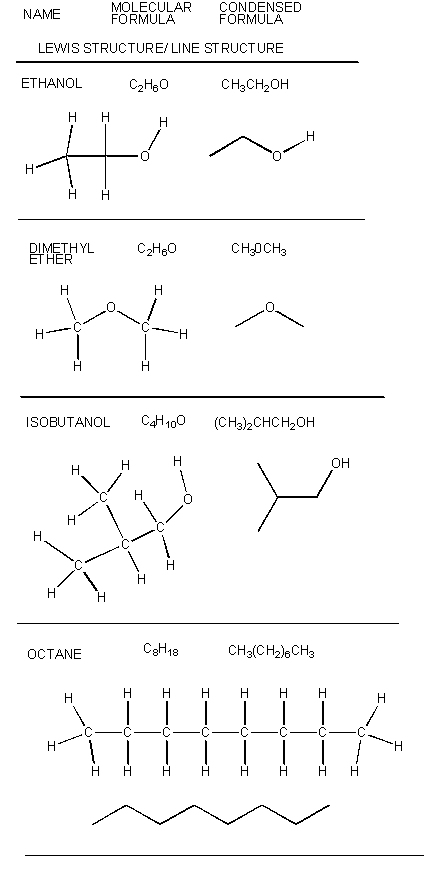
Organic chemistry centers on the chemistry of carbon-containing molecules, which include almost all the molecules within a biological organism. In this chapter, we will review the chemical and physical properties of basic organic molecules, many of which we have already studied. In contrast to the book, I will deemphasize organic nomenclature involved in the naming of organic molecules.
Structures
Carbon forms 4 bonds to other atoms. Since it is not very large, it can get close to other atoms and form reasonably strong bonds. It can form nonpolar covalent bonds to other C's and H's, to form very long chains. It can also form polar covalent bonds with O and N, which are common in biological molecules. Molecules can be represented in many different ways which contains different amounts of structural information, including
Examples are shown below.
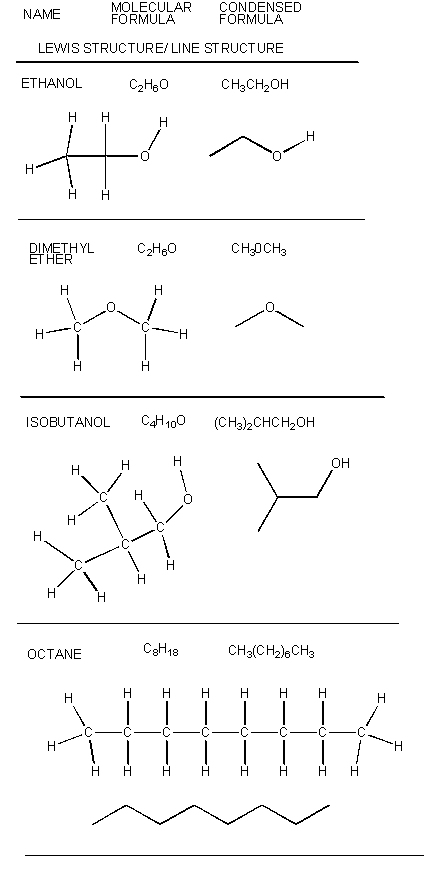
Classes
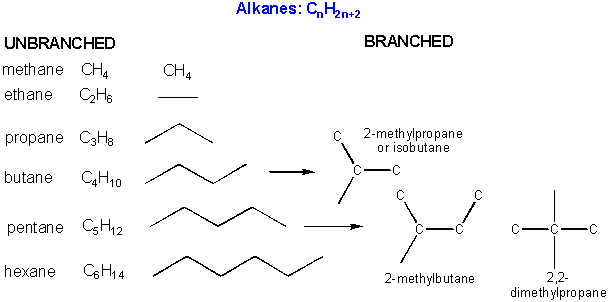
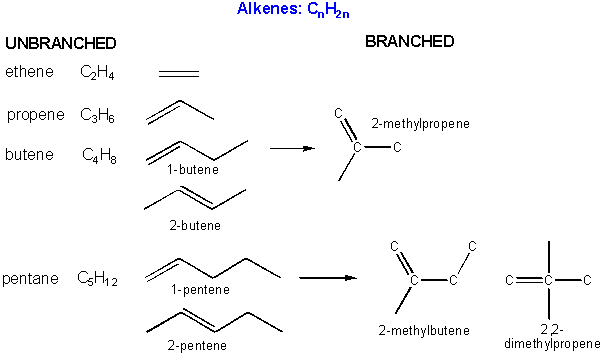
There are millions of different type of organic molecules. Some, composed of just hydrogen and carbon, are called hydrocarbons. Most biologically relevant organic molecules contain other atoms, including O, N, P, S, etc. We have already discussed sugars like glucose, which has the structure C6(H2O)6 - a carbohydrate. Among the hydrocarbons, we will discuss only the simplest - alkanes, which contain C-C single bonds, and alkenes, which have one or more C-C double bonds. Alkanes have the general molecular formula, CnH2n+2, while alkenes with one double bond have the general molecular formula CnH2n. Alkanes and alkenes can contain straight or branched chains of carbon atoms.
Hydrocarbons can also contain carbon chains which connect to each other to form a ring structure, called cycloalkanes or cycloalkenes.
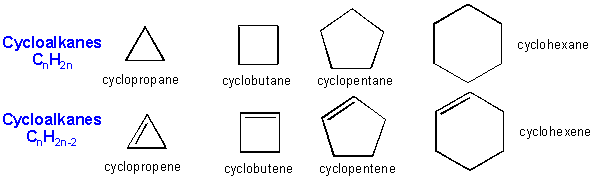
Most organic molecules contain other atoms like O and N, which substitute for a H or C. New chemical functional groups can be created. For example, CH3OH contains a functional group called an alcohol, abbreviated as ROH, where R is a CH3- which is named methyl. Different R groups are shown below:
We will consider an R- group to consist of a group of atoms that end in a C atom which is directly bonded to the adjacent atom shown in the given structure.
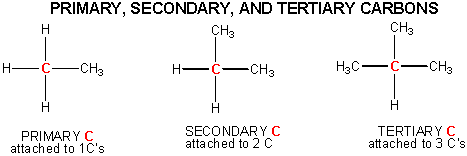
Some carbon chains are linear, some are branched. Carbon atoms in organic molecule can have 1, 2, or 3 carbons attached to them. These are called primary, secondary, and tertiary carbons.
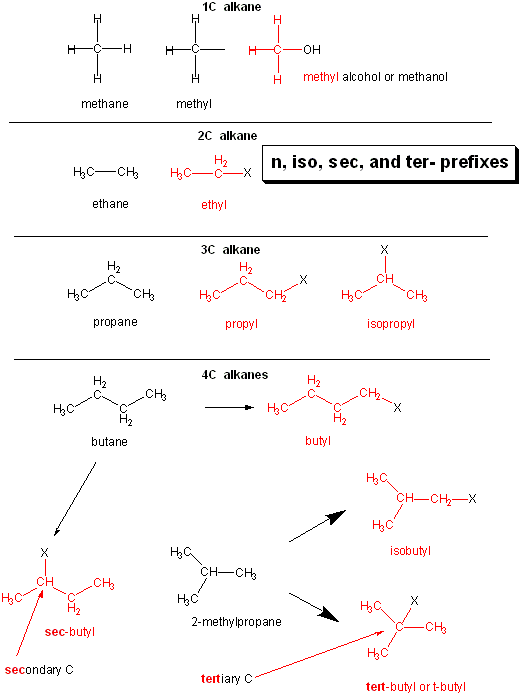
Common names are often used for the alkyl part functional groups as shown below.
Isomers
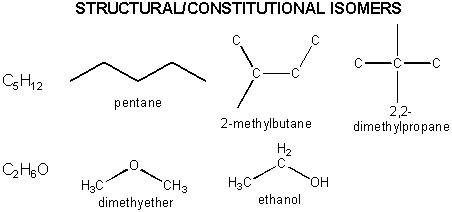
Organic molecules can have the same molecular formula but different structural formulas - i.e. the atoms are connected in different ways. Hence these molecules are not identical - they are different. They are called structural or constitutional isomers . Examples are shown below.
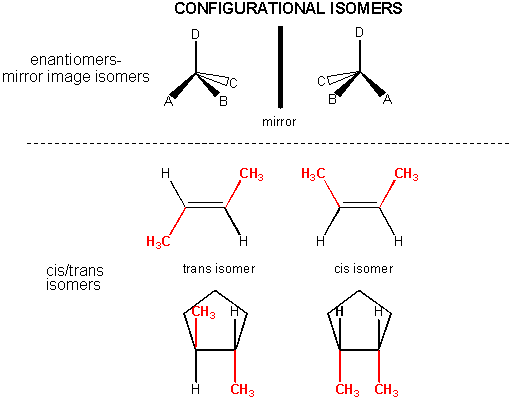
In addition, there are some organic molecules with the same molecular formula and same Lewis structure (hence they have the same connections between atoms in the molecues), but they differ from each other in the 3D - orientation of their atoms in space. They cannot be converted to each other by rotation around single bonds. These are called configurational isomers . Two major examples are shown below.
Breaking Bonds to Carbon
Now its is time to turn to the chemistry of organic moelcules. For hydrocarbons and carbohydrates to react, bonds to carbon atoms must be broken. As we have discussed previously, there are 3 ways to cleave a bond, leading to the formation of unstable, high energy, reactive carbanions, free radicals, and carbocations.

Carbanions are unstable (even though the carbon has an octet) since the negative charge is on a non-electronegative atom. Free radicals and carbocations are both electron deficient - neither have an octet.
As we discussed for acid/base reactions, a C-X bond is more likely to be cleaved if the resulting product (carbanion, free radical, or carbocation) is stablized. Substituents on the C containing the charge or free electron can help stabilize or destabilize the structure. Electronegative substitutents (like F, O, and N) will pulll electrons toward them, which would stabilize the carbanion (by charge dilution) and destabilize the electron poor intermediates (radical and carbocation) by increasing its charge. Large alkyl groups, a source of electron density releases electrons toward the C. This would destabilize the carbanion, but stabilize the carbocation and free radical.

Oxidation Reactions
We have already discussed the oxidation (or combustion) of hydrocarbons and carbohydrates. Examples are shown below:
C6H12O6 + 6O2 -----> 6H2O + 6CO2