
1. Chemical reactions go from reactants to products to the greatest extent when the reactants and products ahve the following properties.

Example: A strong acid (like HCl)
2. If a reaction proceeds to form a charged intermediate and/or charged products, any structural feature of the intermediate or product that stabilizes it will favor the formation of the products.

Example: Acid/Base reactions form charged products; alkenes in addition reactions form carbocation intermediates.
3. Charges on intermediates or products can be stabilized by either:
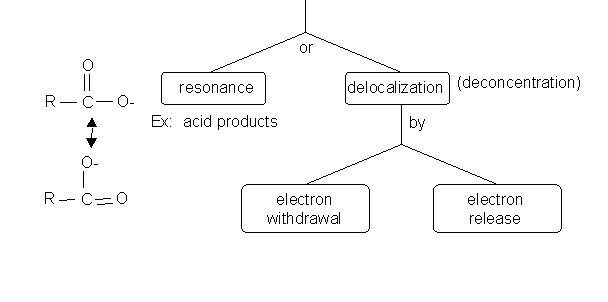
4. Carobocation and carbanion intermediates can be stabilized/destabilized as shown below:
