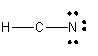
Draw the Lewis Structure of HCN.
1. Draw the skeletal structure showing how the atoms are connected using single bonds. Usually try to draw the most symmetrical structure with the atom of least electronegativity in the center. In this case, you can't really draw a symmetrical structure since there are three atoms. All possible linear structures are shown below.
H-C-N H-N-C C-H-N
Notice that C-H-N is the same as N-H-C just written backwards. ( i.e. they have the same connectivtiy.) You can exclude the last one with H in the middle since H has two bonds and 4 electrons around it. At this point you couldn't differentiate between the first two, so I would give you the connectivity in such a problem, which in this case is H-C-N.
2. Calculate the total number of electrons in the Lewis structure, not the total number of electrons in the actual molecule. Remember, the Lewis structure shows only outer shell electrons (the ones likely to be involved in the reactivity of the atoms involved in forming the molecule. The number of outer shell electrons is obtained from the group number in the periodic table.
| Atom | Gp Number | # outer shell electrons/atom | # atoms | total # electrons in Lewis structure |
| H | 1 | 1 | 1 | 1 |
| C | 4 | 4 | 1 | 4 |
| N | 5 | 5 | 1 | 5 |
|
Total Number of electrons |
10 |
3. Add the remaining electrons to the skeletal structure as nonbonded PAIRS. The skeletal structure already contains 2 bonds or 4 bonded electrons. Hence add 6 additional electrons, as 3 nonbonded pairs, to the structure, giving them to the most electronegative atoms first (in this case N). Don't exceed 8 electrons on any atom (except H which can have only two total electrons). .
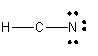
4. Calculate the formal charge of each atom. Do this by assigning electrons to each atoms. Nonbonded electrons are assigned to the atom to which they are attached. For bonded electrons, assign one electron per bond to each atom connected by the bond. If more electrons are assigned than are present in the outer shell of the atom (i.e. the group number), the atom has a negative formal charge (1- if one extra electron, 2- if two extra electrons, etc.). If it has fewer, it is positively charged. Then evaluate the Lewis structure by asking two questions: Does each atom other than H have an octet of electrons? Are the formal charges on the atoms minimized as much as possible?
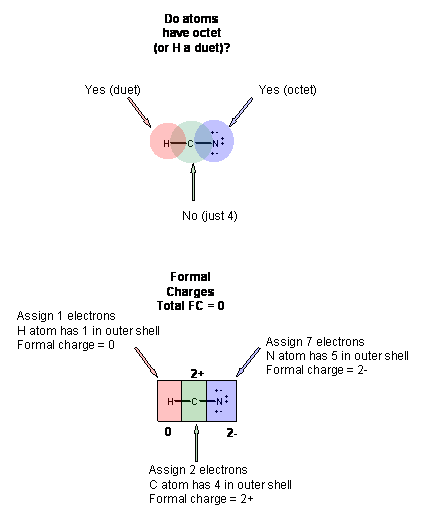
This structure in (3) is clearly is not a good Lewis structure. Hence, you must continue the process.
5. Form bonds to atoms that are deficient in electrons by moving nonbonded electrons pairs from atoms that have an octet. This doesn't cause the atom with the octet to no longer have an octet since the bonded pair is consider as part of both atoms when determining if the atoms connected by the new bond have octets.
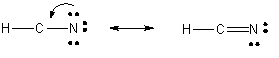
6. Now, reevaluate the next structure as in 4.
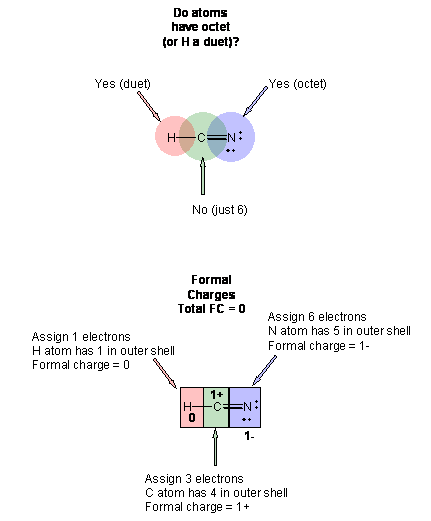
This structure in (5) is clearly is not a good Lewis structure. Hence, you must continue the process by forming another bond to the C.
![]()
7. Re-evaluate the structure as in 4.
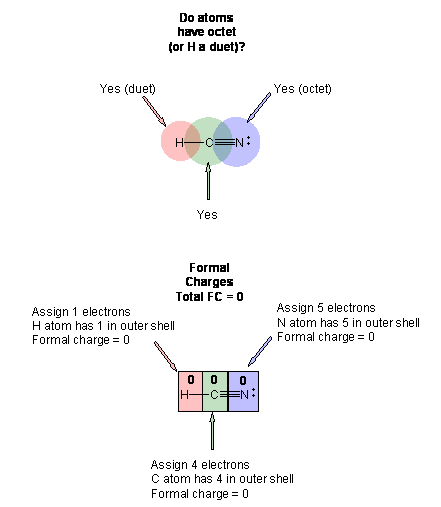
The final structure shows a triple bond between the C and N. All atoms have a formal charge of 0, and the sum of all oxidation numbers for the atoms is equal to the net charge on the molecule. All atoms (except H) have an octet.