7s
7p: n=7, l=1, ml=-1,0,1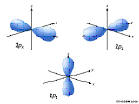
7d: n=7 l=2, ml=-2,-1,0,1,2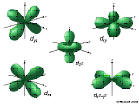
7f:n=5, l=3,ml=-3,-2,-1,0,1,2,3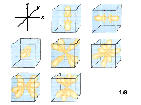
6s
6p: n=6, l=1, ml=-1,0,1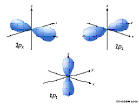
6d: n=6, l=2, ml=-2,-1,0,1,2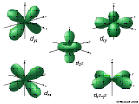
6f:n=5, l=3,ml=-3,-2,-1,0,1,2,3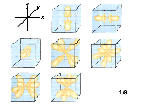
5s
5p: n=5, l=1, ml=-1,0,1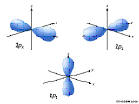
5d: n=5, l=2, ml=-2,-1,0,1,2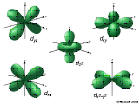
5f:n=5, l=3,ml=-3,-2,-1,0,1,2,3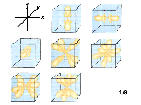
4s
4p: n=4, l=1, ml=-1,0,1
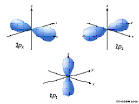
4d: n=4, l=2, ml=-2,-1,0,1,2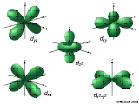
4f:n=4, l=3,ml=-3,-2,-1,0,1,2,3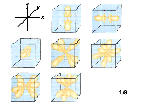
3s: n=3, l=0, ml=-0
3p: n=3, l=1, ml=-1,0,1
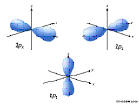
3d: n=3, l=2, ml=-2,-1,0,1,2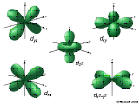
.
2s: n=2, l=0, ml=0
2p: n=2, l=1, ml=-1,0,1
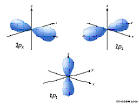
.
.
1s: n=1, l=0
.
.
.
