Biochemistry Online: An Approach Based on Chemical Logic

 Homework
Problems - Literature Learning Module
Homework
Problems - Literature Learning Module
Phosphorylation of Cytochrome c Threonine 28 Regulates Electron Transport Chain Activity in Kidney: Implications for AMP Kinase
Assessment of Biochemistry/Molecular Biology (BMB) Foundational Concepts
Henry Jakubowski, Ph.D., Professor, Chemistry Department, College of Saint Benedict/Saint John's University
This page contains assessment/exam questions using data, figures, and graphs from research journals such as the Journal of Biological Chemistry which allow their use, or from journals such as from PLOS that are completely open access The papers and topics chosen were selected to assess student understanding of the American Society for Biochemistry and Molecular Biology (ASBMB) foundational concepts and learning objectives as well as MCAT2015 foundational concepts and objectives. These two sets of standards broadly overlap. Both ASBMB and the MCAT2015 strongly emphasis scientific inquiry and reasoning skills, which are perhaps best assessed by open-ended questions derived from the literature in which students must employ higher level Bloom skills of application and analysis.
These questions can also be used by students who seek more opportunities to practice interpreting research literature results. The ability to apply, analyze, and evaluate information and concepts are at the heart of scientific inquiry and reasoning skills which are central to the new ASBMB and MCAT2015 competency standards. The questions in this learning module are designed to assess these competencies using open-ended responses instead of multiple-choice questions. Answers can be found at the link at the bottom of this page.
Research Paper: Phosphorylation of Cytochrome c Threonine 28 Regulates Electron Transport Chain Activity in Kidney: Implications for AMP Kinase. Gargi Mahapatraa, Ashwathy Varughesea, Qinqin Ji, Icksoo Leea, Jenney Liua, Asmita Vaishnavb, Christopher Sinklera, Alexandr A. Kapralove, Carlos T. Moraesf, Thomas H. Sandersong, Timothy L. Stemmlerh, Lawrence I. Grossmana, Valerian E. Kagane, Joseph S. Brunzellei, Arthur R. Salomonj, Brian F. P. Edwardsb and Maik Hüttemanna. J. Biol. Chem. 292, 64-79, January 6, 2017
Directly from the introduction: "Cytochrome c (Cytc) is a small (12-kDa) globular nucleus-encoded mitochondrial protein containing a covalently attached heme group with multiple functions. In the electron transport chain (ETC), it functions as a single electron carrier between bc1 complex (complex III) and cytochrome c oxidase (CcO, complex IV) and is thus essential for aerobic energy production. The second important role of Cytc is seen under conditions of stress, when it functions as a crucial pro-apoptotic signal (1). During apoptosis, Cytc is released from mitochondria into the cytosol, where it interacts with Apaf-1 to form the apoptosome, which in turn activates caspase-9 and the downstream executioner caspase cascade. Furthermore, Cytc functions as a cardiolipin peroxidase during the early phase of apoptosis, when it oxidizes the mitochondrial membrane lipid cardiolipin, thereby facilitating its own release from the inner mitochondrial membrane. In contrast, under healthy, non-apoptotic conditions, Cytc acts as a scavenger of reactive oxygen species (ROS) , and it takes part in other redox reactions inside mitochondria, including redox-coupled protein import and reduction of p66Shc, a protein that is implicated in the generation of ROS and apoptosis. Given the multiple functions of Cytc, it is not surprising that it is tightly regulated." These questions deal with its regulation.
1. The investigators purified bovine kidney cytochrome C, done in the presence of phosphatase inhibitors, from bovine kidney. Figure A below shows electrophoretic (PAGE) results for the purification as described. Lane 1, purified kidney Cytc; lane 2, commercial cow heart Cytc; lane 3, ovalbumin; lane 4, EGF-treated A431 (a human carcinoma cell line) total cell lysate. Top, Coomassie gel; bottom, anti-phospho-Thr Western blot. The results are shown below.
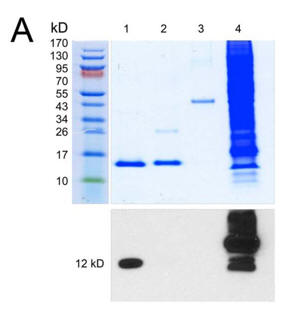
a. Why did the investigators include commercial cytochrome C and ovalbumin in their electrophoresis experiment?
b. Interpret the results of the Western blot. What conclusions can you draw?
One tryptic peptide fragment of cytochrome C that was phosphorylated has this sequence: HKTGPNLHGLFGR. To study if this fragment is phosphorylated, the investigators performed a mass spectral analysis (review). The figure below shows the fragments and labeling systems used to denote MS/MS fragmentation peaks.
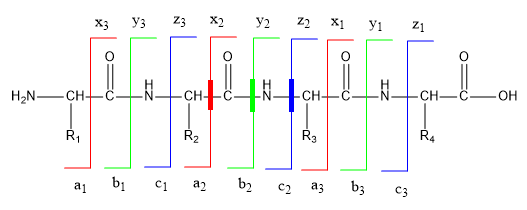
c. What is the MW of the unphosphorylated peptide? Use the ExPASy pI/MW tool to determine it.
d. The phosphorylation site was unambiguously assigned by fragment ions y10 and y11. Use the MW tool above to determine the MW of the dephosphorylated y10 and y11 peaks (since the actual data is not available). Which amino acid is phosphorylated?
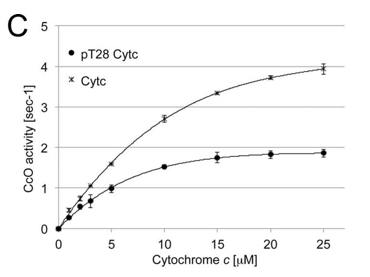
e. The in vivo activity of phosphorylated cytochrome C oxidase (CcO) was measured. What are the substrates for the enzyme? A graph of CcO activity vs [Cytochrome C] is shown below. What effect does phosphorylation have on the activity of the enzyme?
f. A high resolution PAGE gel of the purified cytochrome C shown in the absence (lane 1) and presence (lane 2) of an added nonspecific phosphatase can be seen in Figure D below. Western blotting with an anti-phosphothreonine antibody is shown in the below panel. What do the bands show? What percent (approximate) of the purified cytochrome C is phosphorylated? Why is the result important for the physiological role of the post-translational modification?
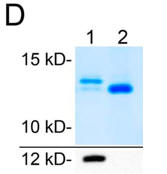
e. If cytochrome C oxidase is inhibited by phosphorylation in vivo, what effect would this have on oxidative phosphorylation? Formation of reactive oxygen species (ROS)?
2. Site specific mutagenesis allows changes in single amino acids in a protein sequence to determine the functional properties of the amino acid.
a. if phospho-Thr 28 (pT28) is important for regulation of CcO, to which amino acid would you mutate T28 so that the mutant would have the same effect on CcO as pT28? Explain.
b. Investigators made the following mutants, T28E and T28A. Why did the makes these mutants? What activities might they have. Would they be phosphorylated in vivo?
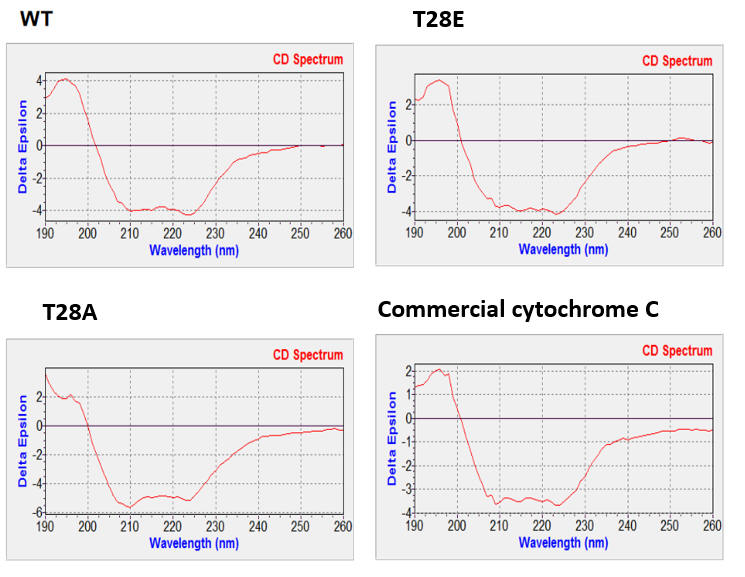
c. Circular dichroism spectra of wild type (WT), mutants, and commercial cytochrome C were determined (shown below). Why did they perform these experiments? What did they show?
d. The WT and mutant proteins were expressed in E. Coli. Would you expect them to be phosphorylated in E. Coli?
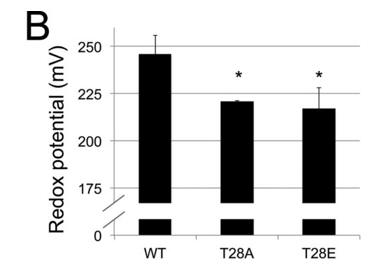
e. The standard reduction potentials of the WT and mutant enzymes are shown below. The mutants' values are similar to that of phosphorylated cytochrome C. Are the mutants better or worse oxidants than the WT?
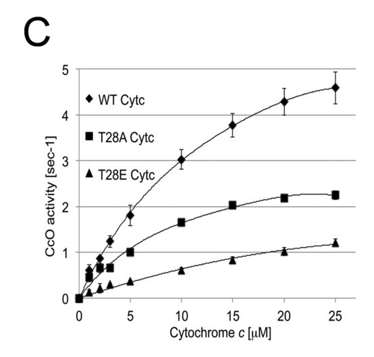
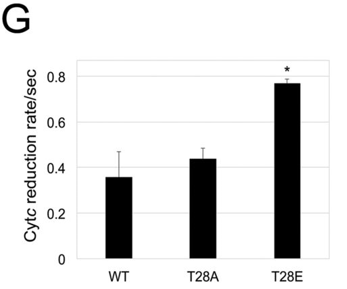
f. The activity of the WT and mutants were examined in an in vitro assay with CcO. The results are shown in C below. Describe the similarities between T28E and the natural pT28 versions of cytochrome C. What might account for the approximately 20% higher in vitro activity of the purified pT28 cytochrome C?
g. Cytochrome C can gain or lose electrons at its heme as well as be oxidatively modified within the protein structure itself. As such it can act as a ROS scavenger. Describe how cytochrome C might react with superoxide, O2-? hydrogen peroxide, H2O2.
h. Figure E below shows results when ferro-(Fe2+)-Cytc variants were treated with low levels (100 μm) of the ROS H2O2. Which reacted with H2O2 faster? Which are more protected from reaction with the ROS? At high levels of H2O2 (3 mM), damage can occur to the protein, which can be monitored by changed in heme absorbance (Fig F below). Comparing the T28A and T28E mutants, what structural feature of T28E might protect it from reaction with H2O2 as evidence by both Figures E and F?
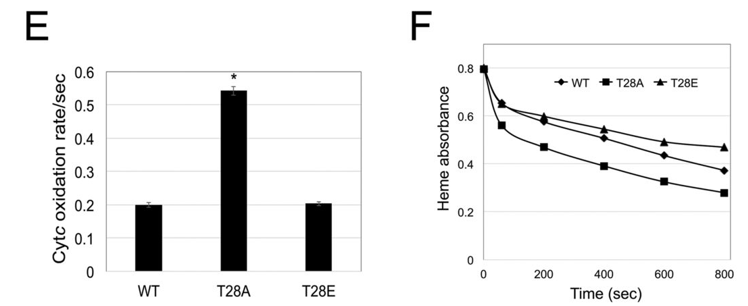
i. Ferri-(Fe3+)-Cytc variants were reduced in the presence of 200 μm ascorbate. The results are shown below.
Which form is a better scavenger of electrons?
.
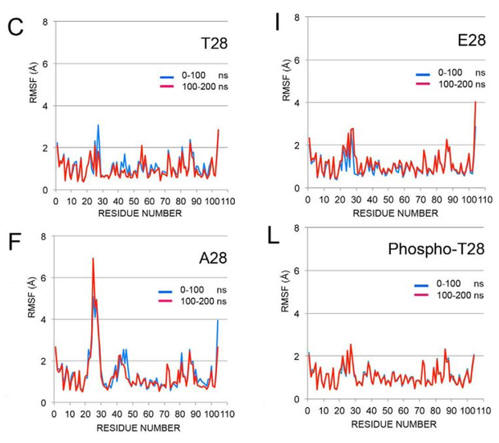
3. Crystal structures of the WT and mutants shown them to be almost identical. To explore possible differences in their solution structures, molecular dynamics simulations were performed on the three protein structures as well as on a model of phosphorylated Cytc with the phosphate group added to Thr28 in the WT chain. The figure below shows RMSF (a measure of the average atomic mobility) of backbone atoms at 0-100 nanoseconds of simulation and 100-200 ns. What structural information can you derive from these results that could account for the results described above.
4. To test the effect of phosphomimetic substitution of Cytc in intact cells on mitochondrial parameters, the investigators generated cell lines stably transfected with empty vector control and WT, T28E, and T28A Cytc, using mouse lung fibroblasts in which both the somatic and testes-specific Cytc isoforms have been knocked out. Determination of cell growth showed that WT and T28E Cytc-expressing cells grew equally well, whereas T28A Cytc-expressing cells showed a 27% reduced growth rate versus WT.
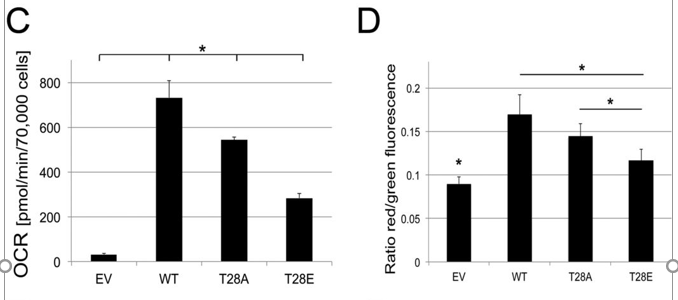
a. Figure C below shows the oxygen consumption rate (OCR) for cells tranfected with an empty vector (EV), WT or mutants. Are the results in C consistent with results described above? Figure D shows the signals from a probe, JC-1, whose fluorescence changes with the transmembrane potential across the inner membrane, ΔΨm. Explain these two results.
b. What effect would expect on the rate of production of ROS by complex I and III in the cells transfected with the T28E mutant? On ATP levels? Explain.
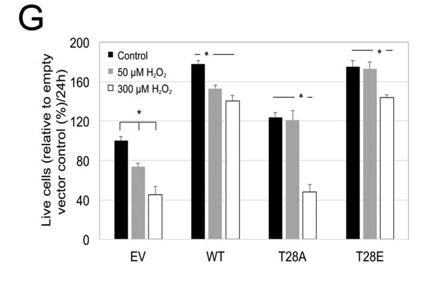
c. To test whether cells expressing T28E Cytc are better protected from H2O2 exposure, cell viability was determined after treatment with H2O2. What would you expect?
d. The actual results are shown below. Explain the results. Are they consistent with your answer above?
5. What kinase phosphorylates T28 in vivo?
a. List some and suggest a candidate whose activity might be important in energy regulation (see Chapter 9).
b. Use Scan Site 3 to predict what might phosphorylate T28. Use P62894 for the accession number for bovine cytochrome C and low stringency. (T28 will be referred to as T29 with the N-terminal Met in the newly synthesized protein given the number 1).
c. To see if AMP Kinase (AMPK) phosphorylates cytochrome C, investigators added AMPK and ATP to bovine heart cytochrome C. The results of PAGE gels and Western blots are shown below. What is the role of ATP in Figure A? Figure B was performed in the presence of ATP and of AMP. What is the role of AMP? Interpret the results.
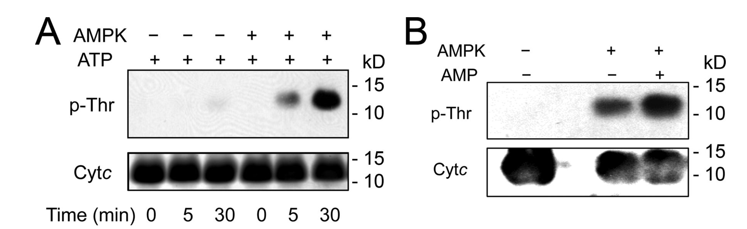
d. MS analysis show the modification to occur on T28. What effect might have occurred if increasing amounts of T28A or T28E were added to the reaction?
7. Does AMPK act as shown above in kidney cells? Investigators added an activator (A769662) and an inhibitor (Compound C) of AMPK in mouse kidney tissue and looked at the effects on phosphorylation of Cyto C (Figure D) and mitochondrial respiration (Figure E). Interpret the results. IP stands for immunoprecipitation. In this case, an antibody against CytC was added to precipitate it. WB stands for Western Blot.
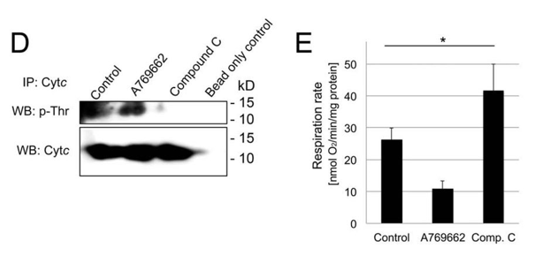
Summarize the results of this paper.
Answers: Literature Learning Module: Cytochrome C regulation of Ox-Phos
Navigation
Return to Biochemistry Online Table of Contents

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.