Biochemistry Online: An Approach Based on Chemical Logic

 Homework
Problems - Literature Learning Module: Transpeptidase
Inhibition
Homework
Problems - Literature Learning Module: Transpeptidase
Inhibition
Assessment of Biochemistry/Molecular Biology (BMB) Foundational Concepts
Henry Jakubowski, Ph.D., Professor, Chemistry Department, College of Saint Benedict/Saint John's University
This page contains assessment/exam questions using data, figures, and graphs from research journals such as the Journal of Biological Chemistry which allow their use, or from journals such as from PLOS that are completely open access The papers and topics chosen were selected to assess student understanding of the American Society for Biochemistry and Molecular Biology (ASBMB) foundational concepts and learning objectives as well as MCAT2015 foundational concepts and objectives. These two sets of standards broadly overlap. Both ASBMB and the MCAT2015 strongly emphasis scientific inquiry and reasoning skills, which are perhaps best assessed by open-ended questions derived from the literature in which students must employ higher level Bloom skills of application and analysis.
These questions can also be used by students who seek more opportunities to practice interpreting research literature results. The ability to apply, analyze, and evaluate information and concepts are at the heart of scientific inquiry and reasoning skills which are central to the new ASBMB and MCAT2015 competency standards. The questions in this learning module are designed to assess these competencies using open-ended responses instead of multiple-choice questions. Answers can be found at the link at the bottom of this page.
Research Paper: A Novel, Species-specific Class of Uncompetitive Inhibitors of gamma-Glutamyl Transpeptidase. Jarrod B. King, Matthew B. West , Paul F. Cook , and Marie H. Hanigan. J. Biol. Chem., 284, NO. 14, pp. 9059–9065, April 3, 2009.
From Abstract: Expression of gamma -glutamyl transpeptidase (GGT) in tumors contributes to resistance to radiation and chemotherapy. GGT is inhibited by glutamine analogues that compete with the sub- strate for the gamma -glutamyl binding site. However, the glutamine analogues that have been evaluated in clinical trials are too toxic for use in humans. We have used high throughput screening to evaluate small molecules for their ability to inhibit GGT and have identified a novel class of inhibitors that are not glutamine analogues.
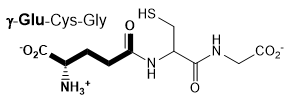
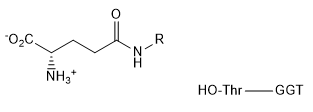
The enzyme γ-glutamyl transpeptidase (GGT), which is localized to the cell surface, cleaves the γ-glutamyl amide bond of extracellular glutathione (right), and transfers the γ-glutamyl group to an acceptor substrate.
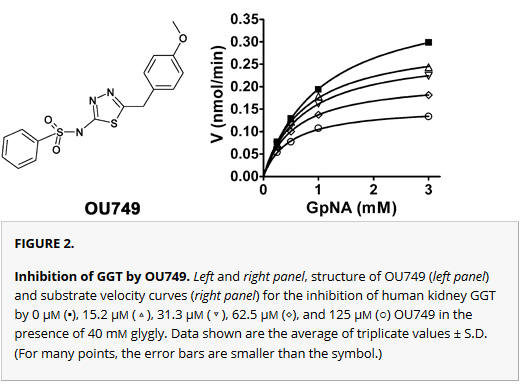
a. A compound, OU749 was discovered that inhibited the enzyme. Shown are the structure of OU749 (left) and substrate velocity curves (right) for the inhibition of human kidney GGT by 0 μm (▪), and various concentration of OU749 (15.2 μm (next curve down), then 31.3 μm, 62.5 μm, and 125 μm) in the presence of 40 mm glygly. From inspection of the graph, do you think the inhibition is competitive? Explain?
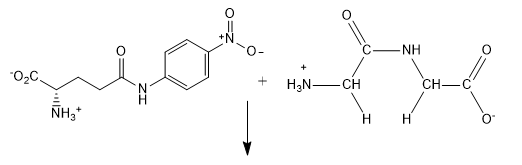
b. b. For the reaction of L-glutamic acid γ-4-nitroanilide HCl (GpNA) and 40 mm glycyl-glycine (glygly) (acceptor), draw the products of the reaction.
c. What wavelength would you choose to monitor the course of the reaction? _______ nm
d. The mechanism seems to involve a covalent intermediate of the substrate with the enzyme through an N terminal Thr. This threonine is conserved in human, rat, mouse, and pig. Draw a mechanism showing the initial reaction of glutathione (abbreviated structure shown below) with the N terminal Thr and then in the subsequent reaction with the acceptor Gly-Gly (represented in your diagram as R’NH2)
e. Site-directed mutagenesis of human GGT has identified
four additional amino acids that are essential to GGT activity Arg 107,
Asp-423, Ser-451, and Ser-452 . All four of these amino acids are identical
in human, rat, mouse, and pig.
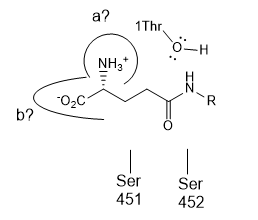
- Which amino acid might likely be found at (a)?
_____
- Which amino acid might likely be found at (b)? _____
- What
type of catalytic mechanism best describes the role of Thr 1 ? _____
-
What type of catalytic mechanism best describes the role of Ser 451 and 452?
f. Complete the Cleland diagram below to show the likely kinetic
mechanism based on your answers from above. A = GPNA, B = Gly-Gly. What are
P and Q?
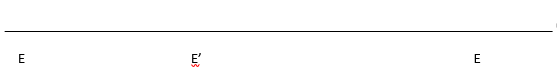
g. g. Kinetic analyses of GGT inhibition by OU749 are shown in the graphs to the right. Shown are double reciprocal plots of the initial velocities of human kidney GGT at varying GpNA (A) and Gly-Gly (B) in the presence of 40 mm glygly (left) or 3 mm GpNA (right) with the following different fixed amounts of inhibitor OU749: 0 μm (▪), 15.2 μm (▴), 31.3 μm (▵), 62.5 μm (⋄), and 125 μm (○). What types of inhibition are show in Graph A? Graph B?
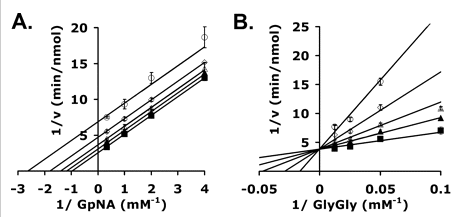
Kinetic Analysis of GGT inhibition by OU749. A–D, double reciprocal plots of the initial velocities of human kidney GGT (A and B) in the presence of 40 mm glygly (A)) or 3 mm GpNA (B) with 0 μm (▪), 15.2 μm (▴), 31.3 μm (▵), 62.5 μm (⋄), and 125 μm (○) OU749 (curves move upward with increasing inhibitor). Data shown are average of triplicate values ± S.D. (For many points, the error bars are smaller than the symbol.)
h. In multi--substrate reaction, the following effects of dead-end inhibitors (I) are observed
-
Slope changes when I and S bind to the same form of the enzyme OR I binds to a form of E (on the horizontal line) which is connected to the form that S binds, and I binds first (for example, I binds to E and then S binds).
-
Y Intercept changes when: I and S bind to different forms of the enzyme unless I binds first and the binding of I and S are in rapid equilibrium
Complete the following with the word same or different
based on your previous answers and knowledge. OU749 binds to a (the)
____________ form of enzyme as GpNA.
OU749 binds to a (the)
____________ form of enzyme as GlyGly
i. Under what conditions do uncompetitive inhibitors lead to more inhibition in vivo than competitive inhibition? (one phrase).
j. The plain old amino acid serine was added to the
enzyme. What type of inhibitor might it be? ________ Show this by
positioning Ser into the active site cartoon cartoon diagram below.
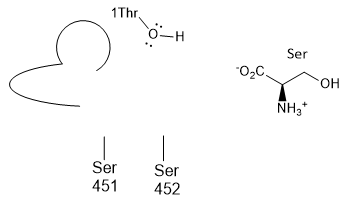
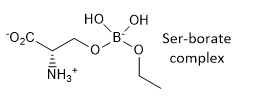
k. A Ser-borate complex (right) is a much more effective
inhibitor. From your knowledge of how enzymes catalyze chemical reactions,
explain why in a single phrase.
k. Site specific mutagenesis was performed to see how changing specific serines (S) would affect activity. (WT = wild-type, unmutated control). The following mutants, denoted as S###X where X is the new amino acid (blacked out in the table below) were made. Which amino acid would the investigators choose to replace Ser in the mutants? ________
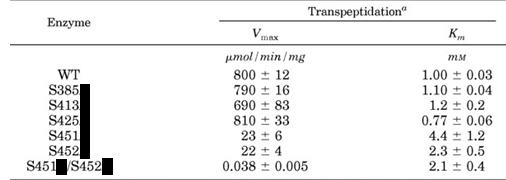
m. Only one kinetic parameter is significantly changed (think of the % changes on the parameters). What step in catalysis is affected in the mutants?
Answers: Literature Learning Module: Transpeptidase
Navigation
Return to Biochemistry Online Table of Contents

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.