Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
B: More Complex Carbohydrates
03/15/16
|
Learning Goals/Objectives for Chapter 3B: After class and this reading, students will be able to
|
This chapter on complex carbohydrates (glycans/glycoconjugates) will review those features that are deemed especially important for a one semester course dealing with structure and function of biomolecules.
B1. Polysaccharides
These contain many monosaccharides in glycosidic links, and may contain many branches. They serve as either structural components or energy storage molecules. The most common polysaccharides consisting of single monosaccharides are:
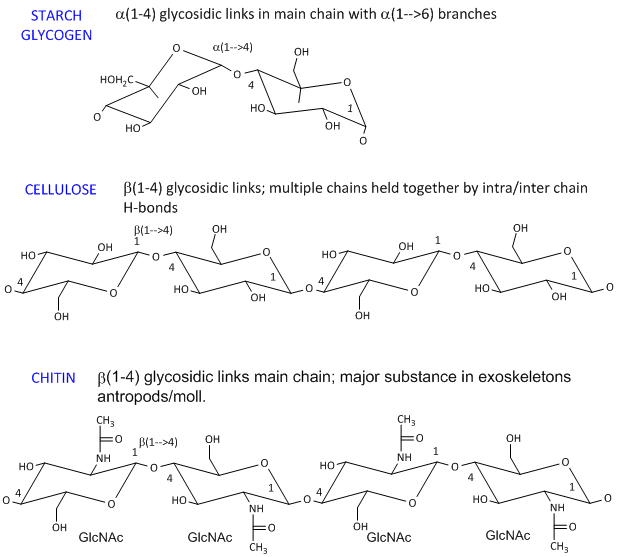
- starch (found in plants). It's a polymer of Glc linked in a main chain through a 1->4 links with a 1->6 branches. Amylose is starch with no branches, while amylopectin has branches. Starch granules consist of about 20% amylose and 80% amylopectin.
-
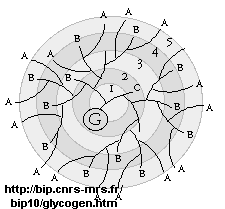
glycogen, the main CHO storage in animals. Muscle and liver glycogen consists of Glc residues in a 1->4 links with lots of a 1->6 branches (many more branches than in starch). The polymer is synthesized on a protein primer called glycogenin (G), and has a structure shown below (in which only 5 rings of the structure are shown instead of the actual 12. (Mel�ndez-Hevia et al. )
-
dextran is a branched polymer of glucose in a 1->6 links with a 1->4 branches and is used in Sephadex chromatography beads.
- celluose, a structural Glc polymer in plants, consists of b 1->4 links. It is held together by intra and inter-chain H-bonds. It is the most abundant biological molecule in nature.
- chitin, the major substance in exoskeletons of anthropods and mollusks is a b 1->4 linked polymer of GlcNAc.
The basic chemical structures of these homopolymers are shown below.
Homopolysaccharides in Chair Conformations
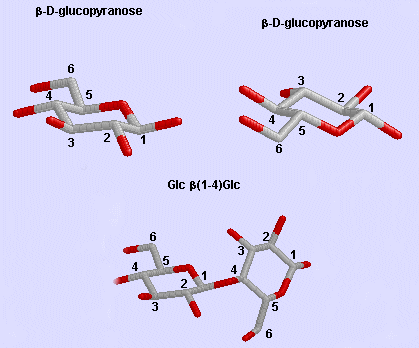
Glc b (1-4) Glc link
It makes great chemical sense to store Glc residues as either glycogen or starch, which is one large molecule. A review of colligative properties would inform you that if all the Glc was stored as the monosaccharide, a great osmotic pressure difference would be found between the outside and inside of the cell. It makes more sense to have glycogen exist as a many-branched linear polymer. When Glc is needed, it is cleaved one residue at a time from all the branches (at the nonreducing ends), producing a large amount of free Glc in a short time.
Phi/Psi angles can also be described for the starch/glycogen main chain (around the acetal O) in a fashion comparable to that for proteins (around the alpha carbon). The phi torsion angle describes rotation around the C1-O bond of the acetal link, while the psi angle describes rotation around the O-C4 bond of the same acetal link, with the glucopyranose ring considers as a rigid rotator (just as the 6 atoms in the planar peptide bond unit). The most extended form of a Glcn polymer occurs when the glycosidic link is b1->4 (as in cellulose), which forms linear chains. The a 1->4 linked main chain of glycogen and starch causes the chain to turn and form a large helix, into which can fit iodine (or I3-), which turns starch purple.
![]() Jsmols:
Jsmols:
![]() Jsmol:
Glycogen
| Jsmol:
Amylose |
Jsmol:
Amylose-2 | Jsmol:
Amylopectin
with I3- | Jsmol:
Amylopectin |
Jsmol:
cellulose
Jsmol:
Glycogen
| Jsmol:
Amylose |
Jsmol:
Amylose-2 | Jsmol:
Amylopectin
with I3- | Jsmol:
Amylopectin |
Jsmol:
cellulose
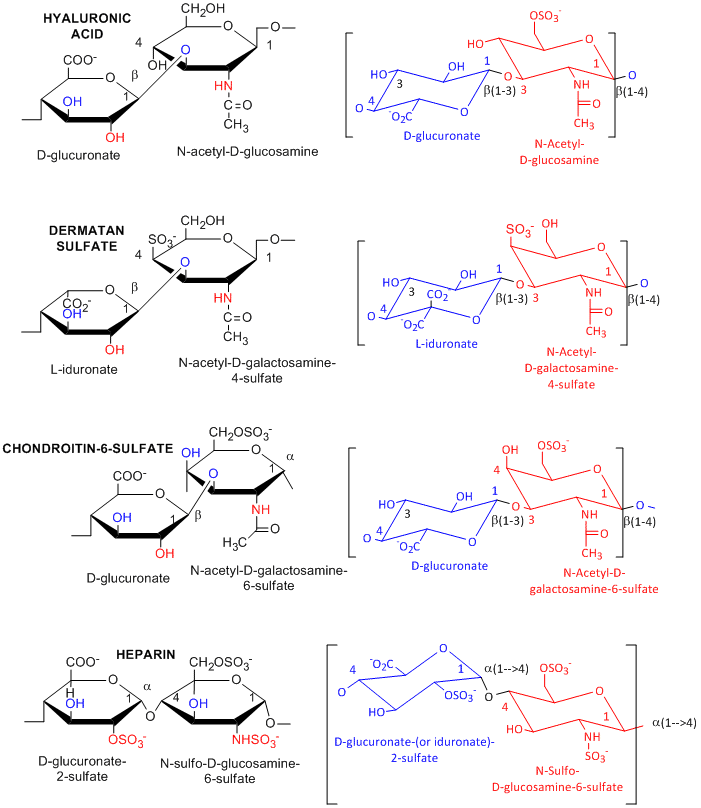
Many polysaccharides consist of repeating dissacharides units. Agarose, a polymer of a disaccharide repeat of (1-->3)-β-D-galactopyranose-(1 --> 4)-3,6-anhydro-α-L-galactopyranose, is often used for a gelable solid phase for electrophoresis of nucleic acid and as a component of chromatography beads. A major class of polysaccharides with dissacharide repeats include the following glycosaminoglycans (GAGs), all which contain one amino sugar in the repeat and in which one or both of the sugars contain negatively charged sulfate or carboxyl groups. The extent and position of sulfation varies widely between and within GAGs.
![]() hyaluronic
acid, a polymer of Glucuronate (b 1->3) GlcNAc: water soluble, in
synovial fluid; backbone for attachment protein, and GAG's
hyaluronic
acid, a polymer of Glucuronate (b 1->3) GlcNAc: water soluble, in
synovial fluid; backbone for attachment protein, and GAG's
![]() dermatan
sulfate, L-iduronate (b 1->3 ) GalNAc-4-sulfate
dermatan
sulfate, L-iduronate (b 1->3 ) GalNAc-4-sulfate
![]() keratan
sulfate, D-Gal (b 1->4) GlcNAc-6-sulfate
keratan
sulfate, D-Gal (b 1->4) GlcNAc-6-sulfate
![]() chondrotin
sulfate, D-glucuronate (b 1->3) GalNAc-4 or 6-sulfate
chondrotin
sulfate, D-glucuronate (b 1->3) GalNAc-4 or 6-sulfate
![]() heparin
- D-glucuronate-2-sulfate (a 1->4) GlcNSulfo-6-sulfate
heparin
- D-glucuronate-2-sulfate (a 1->4) GlcNSulfo-6-sulfate
GAGs are found in the vitreous humor of the eye and synovial fluid of joints, and in connective tissue like tendons, cartilage, etc, as well as skin. They are found in the extracellular matrix and are often covalently attached to proteins to form proteoglycans.
Figure: Glycosaminoglycans
![]()
![]() Jmol:
Heparin
Jmol:
Heparin
A New visual nomenclature for glycobiology
A new symbolic nomenclature for carbohydrates in which monosaccharides are denoted by specific colored geometric shapes has been proposed by the Consortium for Functional Glycomics (2005).
Figure: CHO symbolic nomenclature
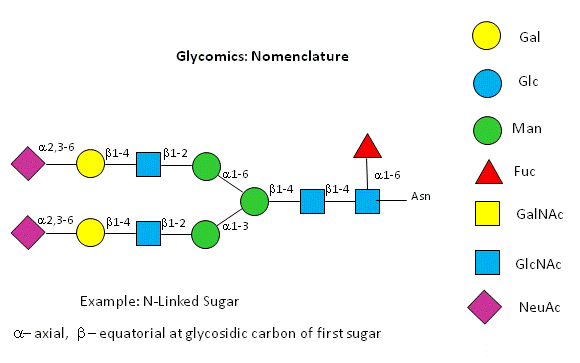
This nomenclature has recently been updated in Appendix 1B of Essentials of Glycobiology, 3rd Edition (Glycobiology 25(12): 1323�1324, 2015. doi: 10.1093/glycob/cwv091 (PMID 26543186)
Navigation
Return to Chapter 3B: More Complex Carbohydrates Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3B: More Complex Carbohydrates

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.