Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
B: More Complex Carbohydrates
03/15/16
|
Learning Goals/Objectives for Chapter 3B: After class and this reading, students will be able to
|
This chapter on complex carbohydrates (glycans/glycoconjugates) will review those features that are deemed especially important for a one semester course dealing with structure and function of biomolecules.
B3. N-linked Glycoproteins
Many proteins, especially those destined for secretion or insertion into membranes, are post-translationally modified by attachment of carbohydrates. They are usually attached through either Asn or Ser side chains. Carbohydrate modifications on the protein appear to be involved in recognition of other binding molecules, prevention of aggregation during protein folding, protection from proteolysis, and increases half-life of the proteins. In contrast to a protein sequence which is determined by a DNA template, sugars are attached to proteins by enzymes which recognize appropriate sites on proteins and attach the sugars. Since there are many sugars which contain many functional groups that can serve as potential attachment sites, the structures of the oligosaccharides attached to proteins are enormously varied, complex, and hence "information rich" compared to linear or folded polymers like DNA and proteins.
a. N-linked Glycoproteins
These contain CHOs attached through either a GlcNAc or GalNAc to an Asn in a X-Asn-X-Thr sequence of the protein. There are three types of N-linked glycoproteins, high mannose, complex, and hybrid. They all contain the same core oligosaccharide - (Man)3(GlcNAc)2 attached to Asn.
Figure: N-linked Glycoproteins
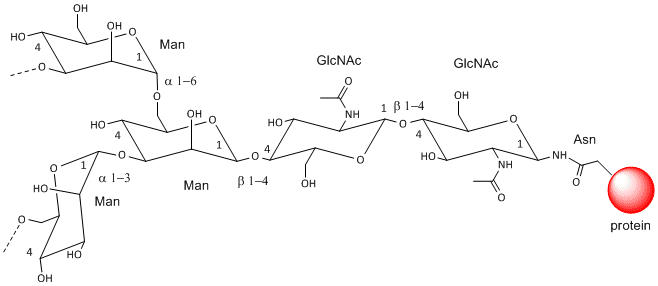
Figure: N-linked high mannose glycoproteins
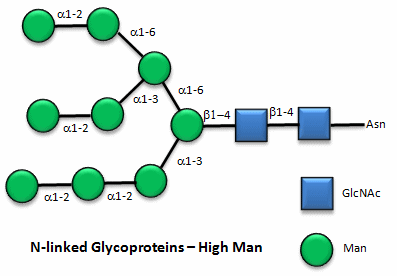
Figure: N-linked complex glycoproteins
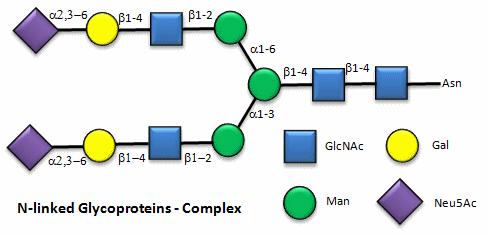
Figure: N-linked hybrid glycoproteins
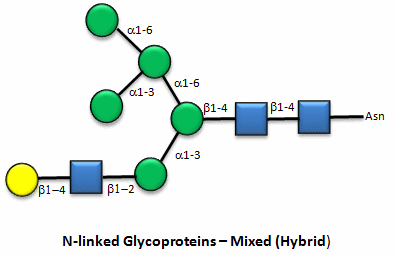
Note that in the hybrid oligosaccharide, one terminus contains Gal(b-1,4)GlcNAc. However, in all other mammals except man, apes, and old world monkey, an additional Gal is often connected in an a-1,3 link to the Gal to give a terminus of: Gal(a-1,3)Gal(b-1,4)GlcNAc. These animals have an additional enzyme, a-1,3 Gal transferase. Bacteria also have this enzyme and since we have been exposed to this link through bacterial infection, we mount an immune response against it. Why is this important? Pig hearts turn out to be similar to human hearts, so they might be good candidates for transplantation into humans (xenotransplants). However, the Gal-a-1,3-Gal link is recognized as foreign, and we mount a significant immune response against it. Several biotech firms are trying to delete the pig a-1,3 Gal transferase which would prevent the addition of the terminal Gal, and make them good donors for transplanted hearts.
![]()
![]() Jmol:
N-linked Glycoprotein
Jmol:
N-linked Glycoprotein
Influenza and the Avian Flu
The influenza virus is a simple yet deadly virus (shown below) . It interacts with human cells through a surface protein, hemagglutinin (HA).
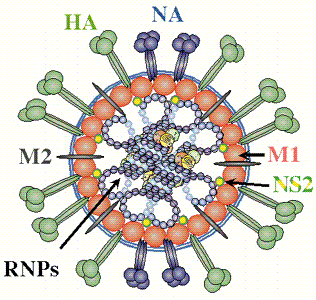
credit: � Paul Digard, Dept Pathology, University of
Cambridge
http://www-micro.msb.le.ac.uk/3035/Orthomyxoviruses.html
The virus binds to host cells through interaction of HA with cell surface carbohydrates. Once bound the virus internalizes, ultimately leading to release of the RNA genome of the virus into the host cell.
![]() Animation:
Influenza entering cell
Animation:
Influenza entering cell
The hemagglutin protein is the most abundant protein on viral surface (as surmised by antibody formation). 15 avian and mammalian variants have been identified (based on antibody studies). Only 3 adapt to humans in last 100 yr, giving pandemic strains H1 (1918), H2 (957) and H3 (1968). Three recent avian variants (H5, H7, and H9) jump directly to humans recently but have low human to human transmissibility.
The influenza hemagglutinin protein has the following characteristics:
- mature form is homotrimer (3 identical protein subunits), MW 220,000 with multiple sites for covalent attachment of sugars. Hemagglutinin is a glycoprotein.
- each monomer synthesized as single polypeptide chain precursor (HA0) that is cleaved into HA1 and HA2 subunits by the protease trypsin in epithelial cells of lung.
- structure known for human (H3), swine (H9), avian (H5) subtypes.
![]()
![]() Jmol:
Hemagglutinin antigen
|
Another View
Jmol:
Hemagglutinin antigen
|
Another View
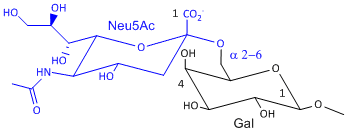
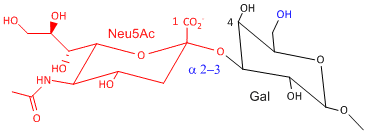
Hemagluttinin bind to sialic acid (Sia), which is covalently attached to many cell membrane glycoproteins. The sialic acid is usually connected through an a(2,3) or a(2,6) link to galactose on N-linked glyocproteins. The subtypes found in avian (and equine) influenza isolates bind preferentially to Sia (a2,3) Gal which predominates in avian GI tract where viruses replicate. Human influenza isolates prefer Sia a(2,6) Gal. Human virus of H1, H2, and H3 subtype (cause 1918, 1957, and 1968 pandemics) recognize Sia a(2,6) Gal, major form in human respiratory tract. The swine influenza HA bind to Sia a(2,6) Gal and some Sia (a2,3) Gal both of which found in swine.
|
Sia a(2,6) Gal (Human) |
Sia a(2,3) Gal (Avian and some Swine) |
|
|
|
|
(made with Sweet, with an OH, not AcNH on sialic acid on C5) |
(made with Sweet, with an OH, not AcNH on sialic acid on C5) |
Structures from: http://www.bme.jhu.edu/~kjyarema/CellSurCarbo1.htm#linkages
The present avian flu (H5N1) is deadly but lacks human to human transmissibility at the moment. Why? One reason is that it appears to bind deep in the lungs and is not released easily on coughing or sneezing. It appears that cell surface glycoproteins deeper in the respiratory tract have Sia (a2,3) Gal which accounts for this pathology.
>The virus, before it leaves the cell, forms a bud on the intracellular side of the cell with the HA and NA in the cell membrane of the host cell. The virus in this state would not leave the cell since its HA molecules would interact with sialic acid residues in the host cell membrane, holding the virus in the membrane. Neuraminidase hydrolyzes sialic acid from cell surface glycoproteins, allowing the virus to complete the budding process and be released from the cell as new viruses. The drugs Oseltamivir (Tamiflu) and zanamivir (Relenza) bind to and inhibit neuraminidase, whose activity is necessary for viral release from infected cells. Tamiflu appears to work against N1 of the present H5N1 avian influenza viruses. Governments across the world are stock piling this drug in case of a pandemic caused by the avian virus jumping directly to humans and becoming transmissible from human to human.
![]() Jmol: Updated
Oseltamivir: Neuramindase N1 complex
Jmol14 (Java) |
JSMol (HTML5)
Jmol: Updated
Oseltamivir: Neuramindase N1 complex
Jmol14 (Java) |
JSMol (HTML5)
Navigation
Return to Chapter 3B: More Complex Carbohydrates Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3B: More Complex Carbohydrates

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.