Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
B: More Complex Carbohydrates
03/15/16
|
Learning Goals/Objectives for Chapter 3B: After class and this reading, students will be able to
|
This chapter on complex carbohydrates (glycans/glycoconjugates) will review those features that are deemed especially important for a one semester course dealing with structure and function of biomolecules.
B5. Proteoglycans
Some proteins are so modified with CHOs that they contain more CHOs than amino acids. Proteins linked to glycosoaminoglycans are together called proteoglycans (PGs). The structures of a few proteoglycans are known. The GAGs are O-linked to the protein, typically to a Ser of a Ser-Gly dipeptide often repeated in the protein. Some of the proteoglycans also contained N-linked oligosaccharide groups.
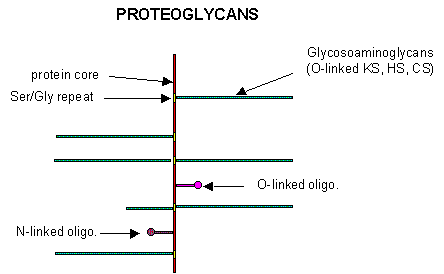
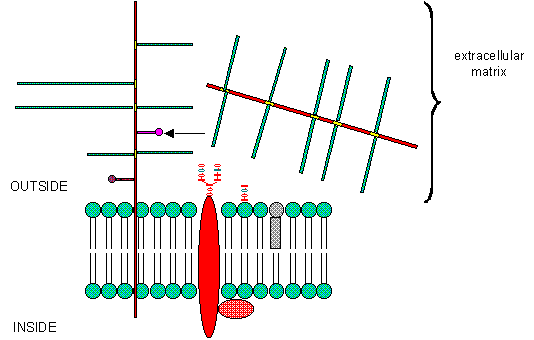
PGs can be soluble and are found in the extracellular matrix, or as integral membrane proteins. Given the diversity of sugars and the varying extent of sulfation, the CHO part of PGs provide an incredible variety of binding structures at or near to the cell surface. One PG, syndecan, binds through its intracellular domain to the internal cytoskeleton of the cell, while interacting with another protein - fibronectin - in the extracelluar matirx. Fibronectin also binds other molecules which can regulate cellular growth and other interactions. PGs act like glue in connecting the extracellular and intracellular functions of the cell. Most proteins bind PGs through a PG binding motif of BBXB or BBBXXB where B is a basic amino acid. Some proteins bind to specific sequences in specific GAGs. For instance, antithrombin 3, an inhibitor of blood clotting, binds specifically to heparin, which enhances its interaction with the clotting protein thrombin.
Navigation
Return to Chapter 3B: More Complex Carbohydrates Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3B: More Complex Carbohydrates

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.