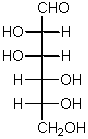
Names:
The definition of a sugar
A 6-member heterocyclic ring containing oxygen and two double bonds within the ring.
The name for this structures suggests
segregation

The name of this structure suggests
symmetry
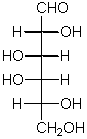
The name of the simplest ketose
CHEMISTRY
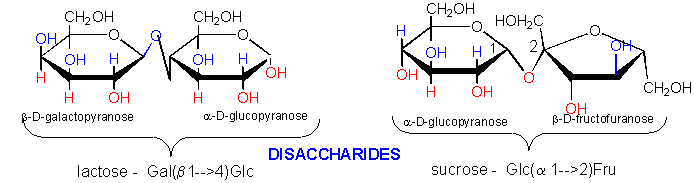
This reaction is typical of aldehydes and nucleophiles
This adds to a hemiacetal to form an acetal.
The disaccharide sucrose, Glc α(1--> 2) Frc can not do this, but the
disaccharide lactose, Gal β(1 -->
4) Glc can.
This reagent and catalyst can be used to cleave acetal links
amide: peptide bond :: acetal: ______
ISOMERS
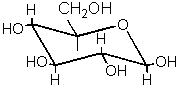
The only aldose with all bulky
substituents in the equatorial position
This pair represents a certain kind of
configurational isomers
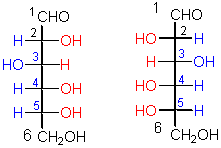
This pair represents a certain kind of
configurational isomers.
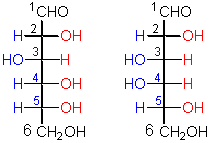
The penultimate C (and last chiral C) contains an OH that points to the right in this kind of sugar.
These two different types of conformers of aldoses are clearly different from configuration isomers.
PROJECTIONS
The dominant form of Glc in solution
The dominate form of Glc in a polysaccharide
The dominant form of fructose in solution
This is the simplest 3C aldose
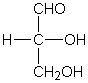
The direction that an -OH group points in a Haworth projection if it points to the right in the aldose or ketose linear form
DERIVATIVES
This sugar contains a phosphate group,
which are common in sugar derivatives as they undergo catabolic degradation in cells.
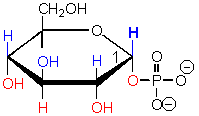
This sugar is in ATP
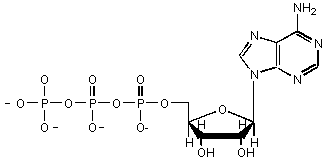
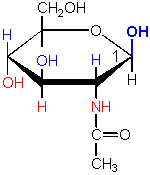
The name of this sugar implies that it is
a sugar-amine derivative
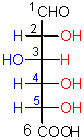
The name of this sugar derivative implies
that it is an acid.
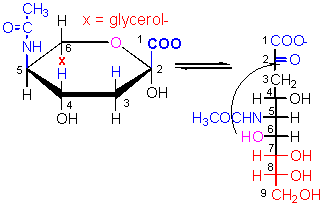
This structure, derived from D-ManNAc and
pyruvic acid, might prove to be the one which most separates human and chimps.
POLYSACCHARIDES
This polysaccharide is used for energy storage in plants
This polysaccharide is used for energy storage in animals.
This polysaccharide is the major constituents of exoskeletons.
This link glycosidic link is found in glycogen and starch
This glycosidic link is found in cellulose.
GAG'S
This is the number of monosaccharides in the repeat unit of glycosoaminoglycans.
This carboxylic acid derivative of glucose is fond in heparin and chondrotin sulfate.
This glycosidic link connects monomers in the dissacharide repeat of heparin.
This glycosoaminoglycan is found in synovial fluid
This is the net charge at physiological pH on the repeat dissacharide unit of heparin.
CELL WALL
Found in bacteria, these structures contain both carbohydrate and amino acids.
Bacteria cell walls contain a repeat of this dissacharide unit.
The link between NAM (MurNAc) on adjacent strands of a bacterial cell wall contains a pentapeptide of this amino acid.
Attached to NAN in Gram + bacterial cell walls, teichoic acid consists of a polymer of this molecule linked by phosphodiester bonds.
The names of two unusual amino acids found in bacterial cell walls.
GLYCOPROTEINS
N-linked oligosaccharides usually connect to a protein at this amino acid.
O-linked oligosaccharides usually connect to a protein at this amino acid.
This sugar is found in all high mannose, N-linked oligosaccharides in glycoproteins.
Complex N-linked oligosaccharides in glycoproteins usually terminate in this sugar derivatives.
This structure is usually found in these
types of N-linked oligosaccharides
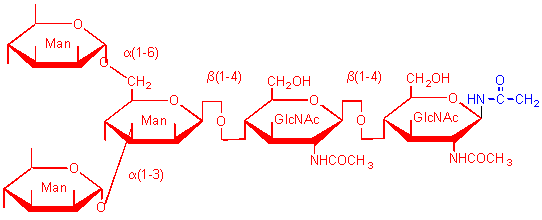
PROTEOGLYCANS AND MEMBRANES
This kind of glycoprotein has an extensive amount of glycosoaminoglycan covalently attached to a protein core.
These two sites are where you would expect to find proteoglycans.
Water-soluble proteins can be attached to membranes by attaching a isoprenoid derivative to this amino acid.
The phospholipid is often attached to GlcNAc in soluble glycoproteins and anchors them to membranes.
Of a eukaryotic, Gram (+) bacteria, or Gram (-) bacteria, the one with the most complicated membrane/cell wall structure.
FINAL JEOPARDY
This structural feature of complex carbohydrates explains why structural determination and synthetic methods to make them have been only recently developed.