Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
B. Lipids in Water
BIOCHEMISTRY - DR. JAKUBOWSKI
2/6/16
Learning Goals/Objectives for Chapter 1B: After class and this reading, students will be able to
|
B1. Micelles and Bilayers
A post-doc once told me that to understand biochemistry and immunology, he had to pretend that he was a molecule. We want to know how lipid molecules, specifically single and double chain amphiphiles, interact with each other and solvent when they are added to water. Before you read the answer, look at the image below and ask yourself the question: What would I do if I were a single chain amphiphile ready to jump into water?
A single chain amphiphile jumps into water!
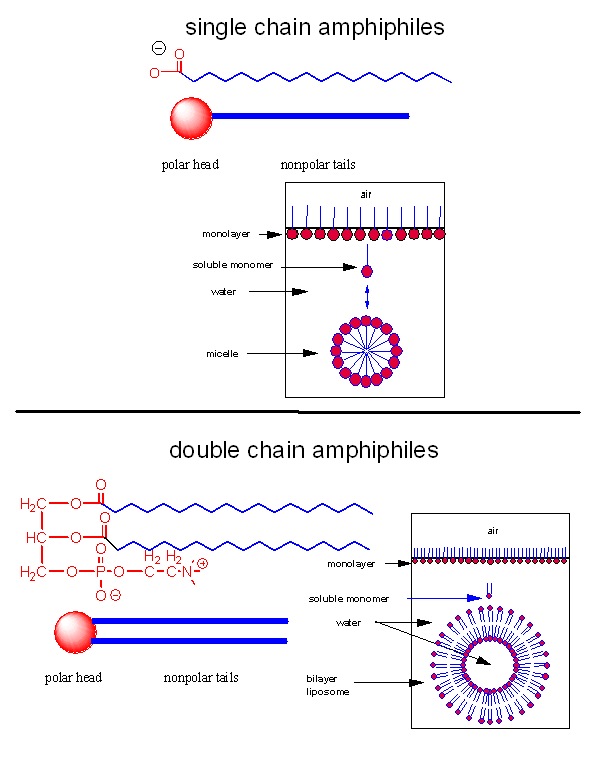
When added to water, single chain amphiphiles form both monolayers on the surface of the water and micelles, while some monomer remain in solution. Double chain amphiphiles form bilayers instead of micelles. (Note: single and double chain amphiphiles can form other multimolecular aggregate structures as well, but these are the most common and are the only ones we will consider. )
Figure: Structures of single and double chain amphipiles in water - Micelles and Bilayers
![]() Jmol
Updated Micelle
Jmol14 (Java) |
JSMol (HTML5)
Jmol
Updated Micelle
Jmol14 (Java) |
JSMol (HTML5)
![]() Jmol: Updated Nonhydrated Bilayer
Jmol14 (Java) |
JSMol (HTML5)
Jmol: Updated Nonhydrated Bilayer
Jmol14 (Java) |
JSMol (HTML5)
The micelle interior is completely nonpolar. Spherical bilayers that enclose an aqueous compartment are called vesicles or liposomes. Micelles and bilayers, formed from single and double-chain amphiphiles, respectively, represent noncovalent aggregates and hence are formed by an entirely physical process. No covalent steps are required. The formation of these structures can be understood from the study of the intermolecular forces (IMFs) involved as well as thermodyamics. First we will review the IMFs involved. Consider the attractive forces. The buried acyl chains can interact and be stabilized by London forces. They are sequestered from water. This view fits our simple axiom of "like-dissolves like". The polar head groups can be stabilized by ion-dipole bonds between charged head groups and water. Likewise H-bonds between water and the head group stabilizes the exposed head groups in water. Repulsive forces may also be involved. Head groups can repel each other through steric factors, or ion-ion repulsion from like-charged head groups. The attractive forces must be greater than the repulsive forces, which lead to these molecular aggregates. One problem arises with this simple explanation. For a micelle or bilayer to form, many monomers must aggregate to form a single micelle or vesicle. This suggests that micelle and vesicle formation should be entropically disfavored!
Common single chain amphiphiles that form micelles are detergents (like sodium dodecyl sulfate - SDS) as well as fatty acids, which themselves are detergents. NaOH feels slippery on your skin since the base hydrolyses the fatty acids esterified to skin lipids. The free fatty acids then aggregate spontaneously to form micelles which act like detergents.
![]() Pre-Class
Questions:
Lipid Structure: B. Lipids in Water - Question
Pre-Class
Questions:
Lipid Structure: B. Lipids in Water - Question
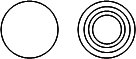
Liposomes produced in the lab can be unilamellar, consisting of a single bilayer surrounding the internal aqueous compartment, or multilamellar, consisting of multiple bilayers surrounding the enclosed aqueous solution. You can image the multilamellar vesicles resembles an onion with its multiple layers. Cartoons of unilamellar and multilamellar liposomes are shown below, where each concentric circle represents a bilayer.
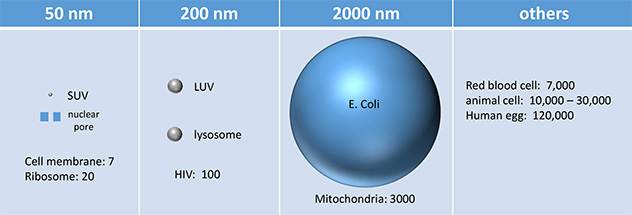
Liposomes vary in diameter. They can be generally categorized into small (S, diameter < 25 nm), intermediate (I, diameter around 100 nm), and large (L, diameter from 250-1000 nm). If these vesicles are unilamellar, they are abbreviated as SUV, IUV, and LUV, respectively. Their various sizes are shown below, in comparison to other large biological structures. In this lab, we will make and characterize LUV.
The chemical composition of liposomes can be widely varied. Most contain neutral phospholipids like phosphatidyl choline (PC), phosphatidyl ethanolamine (PE), or sphingomyelin (SM), supplemented, if desired, with negatively charged phospholipids, like phosphatidyl serine (PS) and phosphatidyl glycerol (PG). In addition, single chain amphiphiles like cholesterol (C) and detergents can be incorporated into the bilayer membrane, which modulates the fluidity and transition temperature (Tm) of the bilayer. If present in too great a concentration, single chain amphiphiles like detergents, which form micelles, can disrupt the membrane so completely that the double chain amphiphiles become incorporated into detergent micelles, now called mixed micelles, in a process which effectively destroys the membrane bilayer.
In lab (for those that take it) you will make liposomes containing only natural PC from fresh egg yolk. Remember, the two fatty acids in naturally occurring phospholipids can be of a multiple of lengths and degrees of unsaturation. The average fatty acid composition of egg yolk PC (average molecular weight = 750) is shown in the table below, obtained from Liposomes: a practical approach, edited by R.R.C. New.
|
Fatty Acid |
%Fatty acid at C1 |
% Fatty Acid at C2 |
|
16:0 |
68.8 |
1.8 |
|
18:0 |
25.8 |
1.2 |
|
18:1 |
4.7 |
48.9 |
|
18:2 |
0.2 |
11.1 |
|
18:3 |
0.5 |
- |
|
20:4 |
- |
2.1 |
|
20:5 |
- |
7.1 |
|
22:5 |
- |
2.6 |
|
22:6 |
- |
25.2 |
-
 Avanti
Polar Lipids (where we get our egg PC):
Fatty Acid Distribution for different tissues
Avanti
Polar Lipids (where we get our egg PC):
Fatty Acid Distribution for different tissues
Given the large degree of unsaturation at C2, what do you expect the transition temperature of a liposome composed only of egg yolk PC to be? (Vesicles made using more saturated PC from mammalian sources have Tm of around 40oC.) This high degree of unsaturation makes egg yolk PC very susceptible to oxidation, which could alter the properties of the liposome dramatically. Synthetic PC made with saturated fatty acids could alleviate that problem.
The properties of liposomes (charge density, membrane fluidity, and permeability) are determined by the lipid composition and size of the vesicle. The desired properties will be, in turn, determined by the use of the particular liposome. The vesicles offer wonderful, simple models to study the biochemistry and biophysics of natural membranes. In fact, membrane proteins can actually be incorporated into the liposome bilayer using the exact method you will be using. But apart from these purposes, liposomes can be used to encapsulate water soluble molecules such as nucleic acids, proteins, and toxic drugs. These liposomes can be targeted to specific cells if antibodies or other molecules which will bind specifically to the target cell can be incorporated into the bilayer of the vesicle. Intraliposomal material may then be transferred into the cell either by fusion of the vesicle with the cell, or by endocytosis of the vesicle.
Navigation
Return to Biochemistry Online Table of Contents
Return to Chapt 1B: Lipids in Water
Archived version of full Chapter 1B Lipids in Water