Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
B: Lipids in Water
BIOCHEMISTRY - DR. JAKUBOWSKI
2/6/16
Learning Goals/Objectives for Chapter 1B: After class and this reading, students will be able to
|
B2. Making Liposomes in the Lab
Since phospholipids will spontaneously form some type of bilayer structure when placed in water, most efforts in liposome production involve producing vesicles with the desired size, lamellar structure, and physical characteristics, which as previously stated is controlled both by liposome size and chemical composition. Also, ways must be developed to entrap the desired molecule inside the vesicle in the most cost-effective manner, and with minimal leaking of contents. All methods of production involve four steps: 1) drying of organic solvent-solubilized lipids; 2) dispersion of the lipids in the appropriate aqueous solution; 3) separation of liposome from excess starting reagents; and 4) characterization of the vesicles for chemical composition, Tm, permeability, size, etc.
1. Drying of lipids:
Purified lipids of the desired composition (often times egg PC:cholesterol:PS in molar ratios of 0.9:1.0:0.1) are dissolved in a purified, water-free organic solvent mixture (often chloroform/methanol, 2:1 v/v) and dried down in a round bottom flask on a rotary evaporator under reduced pressure (using a water aspirator) and slightly elevated temperature (20-40oC). The rapid rotation of the flask will ensure that the lipid is dispersed over a large surface area, and will increase the rate of evaporation. To remove the last traces of solvent, the dried flask is usually placed under a high vacuum overnight. If a small volume (< 1 ml) of lipid solution is used,, the solvent can be evaporated under a stream of nitrogen. To avoid entrapment of residual chloroform in the lipid film, the film is dissolved in t-butyl-methylether diethyl ether , and dried several times. Alternatively, the residual solvent can be removed under high vacuum.
2. Dispersion of the lipids in the appropriate aqueous solution:
There are three main methods of dispersing the lipids into an aqueous solution to form liposomes.
a. mechanical dispersion - in this method, lipid dried onto the inside glass surface of a container is hydrated with an aqueous solution, which literally peals off the lipid to form multilamellar - MLV - (multiple bilayers separated by water) vesicles. Only a small part of the aqueous solution is encapsulated inside the liposome, so this is not the method of choice for the encapsulation of expensive or rather insoluble solutes. Depending on the degree of agitation and the nature of the lipid used, different sized liposomes can be prepared. These multilamellar liposomes can be further processed to form unilamellar liposomes by several techniques. These include probe or bath sonication of the MLV, extrusion at high pressure of the MLV through membrane filters of defined pore size, or pH-induced vesiculation in which a transient change in pH destabilizes the MLV in favor of unilamellar liposomes. Another technique involves fusion of SUV by repeated freezing and thawing or by fusion of SUV containing acidic phospholipids (such as PS) through Ca2+ mediated aggregation.
b. organic solvent dispersion - In these methods, the lipids, which are dissolved in organic solvents, are injected through a fine needle, at a slow rate, into an aqueous solution in which the organic solvent may be miscible (such as ethanol) or immiscible (such as ether). In each case, the lipids orient at the interface between the organic solvent and aqueous solution, to form bilayer structures. Injection of ethanol-dissolved lipids provides a simple way to produce SUV, but because liposome formation can not occur at an ethanol concentration greater than 7.5%, only a fraction of the total aqueous phase can be entrapped in the vesicle; hence this technique is not cost-effective for entrapment of an expensive solute.
Alternatively, the lipid can be dissolved in ether and slowly injected into an aqueous solution which is warmed so that the ether evaporates at the rate at which it is injected. Since the ether is volatilized, large amounts of lipid can be introduced and encapsulation efficiency of the aqueous solution is high.
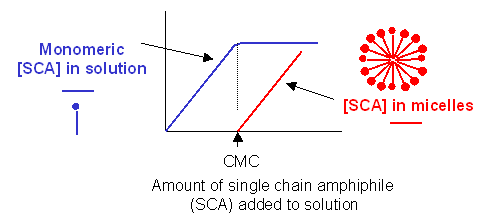
c. detergent dispersion and solubilization - In this method, lipids are solubilized in an aqueous solution through the addition of detergents. The detergents are removed slowly from the solution, resulting in the spontaneous formation of liposomes. Detergents are single chain amphiphiles that spontaneously form micelles in aqueous solution when the concentration of free lipid rises to a minimum critical value, the critical micelle concentration (CMC); at this concentration, self-association of detergent results in the formation of a stable aggregate, the micelle. This is illustrated in the figure below, along with the CMC of several different detergents.
CRITICAL MICELLE CONCENTRATION
|
name |
mM |
mg/ml |
MW |
| n-hepty glucopyranoside | 70 | 19.5 | 278 |
| n-octyl glucopyranoside | 23.2 | 6.8 | 292 |
| n-nonyl glucopyranoside | 6.5 | 2.0 | 306 |
| n-decyl maltoside | 2.19 | 1.1 | 499 |
| n-dodecyl maltotrioside | 0.2 | 0.16 | 825 |
| Triton X-100 (a) | 0.24 | 0.15 | 625 |
| Nonidet P-40 (b) | 0.29 | 0.02 | 603 |
| Tween 20 (c) | 0.033 | 0.04 | 1364 |
| Brij 98 (d) | 0.025 | 0.04 | 1527 |
| sodium deoxycholate | 2-6 | 1.7 | 415 |
| sodium taurocholate | 10-15 | 6.7 | 538 |
| sodium cholate | 14 | 6.0 | 431 |
| sodium dodecyl sulfate | 8.3 | 2.4 | 289 |
Lipid Data from Avanti Polar Lipids
-
 Phase
Transition Temperatures for Glycerophospholipids
Phase
Transition Temperatures for Glycerophospholipids -
 Miscibility
of Phospholipid Binary Mixtures
Miscibility
of Phospholipid Binary Mixtures -
 Ionization
Constants Of Phospholipids
Ionization
Constants Of Phospholipids -
 Critical
Micelle Concentrations (CMC)
Critical
Micelle Concentrations (CMC)
In this procedure, lipid is deposited in a small container. An aqueous solution is then added, containing water-soluble molecules for encapsulation. Detergent is then added at a concentration in excess of the lipid concentration and greater than its CMC. The lipid molecules are then "emulsified" in the detergent micelle. The solubilized mixture is then placed in a semi-permeable dialysis bag, which is placed in a large volume of an aqueous solution. The free detergent in solution is in equilibrium with the detergent in the micelle. The bag contains microscopic holes large enough for the monomeric detergent molecule to pass through, but small enough so that the large micelle can not. The lipid, during this process, is embedded in the micelle forming a detergent-lipid mixed micelle. As dialysis continues, the monomeric detergent partitions throughout both the volume in the bag and the volume surrounding the bag, while the mixed micelle remains in the bag. If the aqueous solution surrounding the bag is changed several times with fresh solution, the equilibrium in the bag is shifted to the monomeric form. Alternatively, detergent-adsorbing beads (such as Bio Bead SM-2 by Bio-Rad) can be placed in the aqueous solution surrounding the bag to speed up the process of detergent reequilibration. Eventually, all the detergent is in this form, and during the process, which occurs slowly, the lipid in the mixed micelle self-associates to form a liposome. A detergent of low monomer molecular weight and a high CMC is most desirable for this method of liposome production. Another method of removing the free detergent is through gel filtration chromatography. In this technique, molecules of disparate molecular weights can be separated from each other. An explanation follows this discussion. This method of formation of unilamellar liposomes is the method of choice if membrane proteins are to be inserted into the liposome bilayer for the purpose of targeting the liposome. It is not the best method, however, for quantitative encapsulation of expensive soluble molecules.
-
 Liposome
preparation from Avanti
Liposome
preparation from Avanti
3. Separation of liposome from excess starting reagents:
Once the liposomes are formed, they must be separated from free monomeric lipid, detergent, and unencapsulated solutes. This can be done again by dialysis, or more readily by gel filtration chromatography. Macromolecules of different sizes can be separated on a column in which the stationary phase is a polymerized agarose or acrylamide bead, which contain pores of various sizes. A small molecule (such as monomer detergent, free lipid, or small aqueous solute) in the mobile phase (aqueous buffered solution) may enter the pores in the bead, while a larger macromolecule or aggregate (such as a large protein, a micelle, or a liposome) may not, due to size restriction. The result is that a larger fraction of the overall volume of the column is available to the smaller molecules, which thus spend a longer time on the column and are eluted by the mobile solvent after the larger species. We will study the theory of gel chromatography in a future experiment.
4. Characterization of the vesicles:
Liposomes can be characterized both chemically, to determine the average lipid and protein makeup of the bilayer, and physically, to determine the size, permeability, lamellarity, and amount of encapsulated material. Size is usually determined by electron microscopy or indirectly by light scattering from these large species. In this lab, instead of characterizing the lipid makeup of the liposome, which you know to be composed only of PC, you will perform thin layer chromatography on a series of phospholipids. You will also determine whether you have encapsulated an solute in the liposome.
Navigation
Return to Biochemistry Online Table of Contents
Return to Chapt 1B: Lipids in Water
Archived version of full Chapter 1B Lipids in Water