Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
B: Lipids in Water
BIOCHEMISTRY - DR. JAKUBOWSKI
2/6/16
Learning Goals/Objectives for Chapter 1B: After class and this reading, students will be able to
|
B3. Variant of Micelles and Vesicles
Many different kinds of amphiphilic molecules can be induced to form aggregates like micelles and bilayers, or to form unique aggregates on designed surfaces. One recent example is the formation of vesicle bilayers of potassium salt of pentaphenyl fullerene (Ph5C60K) in water. The anions form stable spherical vesicles with about 12,000 anions in a structure of average radius of 17 nanometers. The link above shows a bilayer vesicle model with about 12,000 molecules. A sector has been cut out for enhanced visibility. The hydrophobic fullerene bodies are shown in green, the hydrophilic- charged cyclopentadienide regions are in blue.
Transport of fatty acids and other lipids in the blood
If fatty acids are so insoluble in water (and must form micelles to become dispersed in water), how do they move around the blood and be delivered to tissues that need them? There are two main transport vehicles to allow lipid movement in the blood. For free fatty acids released from fat (adipose) tissue after intracellular hydrolysis of triacylglycerides, the fatty acid is bound to the most abundant blood serum protein, albumin. This protein can bind many fatty acids for each albumin protein. We will study protein structure and binding interactions in subsequent chapters.
![]() Updated
Human serum albumin complex with 18:0
Jmol14 (Java) |
JSMol (HTML5)
Updated
Human serum albumin complex with 18:0
Jmol14 (Java) |
JSMol (HTML5)
More complex lipids like triacylglycerides, cholesterol, cholesterol esters, and phospholipids are transported in large vesicles like structures called lipoproteins. These, as their name implies, contain protein (on the surface) and lipids. Since they need to carry nonpolar molecules, their inside contains no water. The outside consists of a monolayer of phosopholipids and cholesterol esters, while the insides contain more nonpolar lipids.
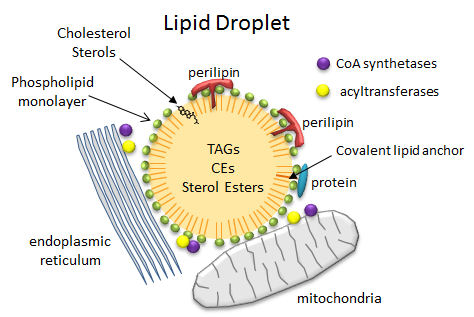
So how are triacylglycerides stored in cells? - Lipid Droplets
In contrast to the common single and double chain amphiphiles, which have charged atoms in their head groups and which form micelles and bilayers, respectively, triacylglycerides (TAGs) and cholesterol esters (CEs), which are almost completely nonpolar, coalesce into lipid droplets in cells. These droplets can range from the very big, which are found in adipocytes (fat cells) where they take up almost all of the available space and where they are used for energy storage, to small, which are found in all cells, where they are used mostly for membrane biogenesis and energy mobilization. When esterified into esters, fatty acids and steroids also pose less potential toxicity to cells. Lipid droplets are often found in close approximation or attachment to mitochondria, endoplasmic reticulum (ER) and peroxisomes (where plasmalogens with ether-linked fatty acid instead of ester-linked are synthesized), all organelles intimately involved in membrane and energy biochemistry. Many of the enzymes (acyltransferases for example) required for TAG metabolism are found in the mitochondria and the ER.
The droplets are now considered actual cellular organelles. In contrast to other organelles which are bounded by a bilayer member, lipid droplets are surrounded by a monolayer of phospholipids which prevents exposure of the nonpolar contents to the aqueous cytoplasm. PC and PE appear to be the major phospholipids in the monolayer and both are synthesized mostly in the ER. There are many different attached proteins, including
- perilipins: There are multiple types of perlipins. Perilipin 1 is found in adipoctyes and cells that synthesize steroid (andrenals, ovaries, and testes). Perilipin 2 and 3 are found in most cells
- Acyl CoA synthetases and acyltransferase: These enzymes activate free fatty acids for metabolic processes.
Figure: Lipid Droplets
Navigation
Return to Biochemistry Online Table of Contents
Return to Chapt 1B: Lipids in Water
Archived version of full Chapter 1B Lipids in Water