Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 2 - PROTEIN STRUCTURE
A: AMINO ACIDS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 02/27/16
|
Learning Goals/Objectives for Chapter 2A: After class and this reading, students will be able to
|
A4. Introduction to Amino Acid Reactivity
You should be able to identify which side chains contain H bond donors and acceptors. Likewise, some are acids and bases. You should be familiar with the approximate pKa's of the side chains, and the N and C terminal groups. Three of the amino acid side chains (Trp, Tyr, and Phe) contribute significantly to the UV absorption of a protein at 280 nm. This section will dealing predominantly with the chemical reactivity of the side chains, which is important in understanding the properties of the proteins. Many of the side chains are nucleophiles. Nucleophilicity is a measure of how rapidly molecules with lone pairs of electrons can react in nucleophilic substitution reactions. It correlates with basicity, which measures the extent to which a molecule with lone pairs can react with an acid (Bronsted or Lewis). The properties of the atom which holds the lone pair are important in determining both nucleophilicity and basicity. In both cases, the atom must be willing to share its unbonded electron pair. If the atoms holding the nonbonded pair is more electronegative, it will be less likely to share its electrons, and that molecule will be a poorer nucleophile (nu:) and weaker base. Using these ideas, it should be clear that RNH2 is a better nucelophile than ROH, OH- is a better than H2O and RSH is a better than H2O. In the latter case, S is bigger and its electron cloud is more polarizable - hence it is more reactive. The important side chain nucleophiles (in order from most to least nucleophilic) are Cys (RSH, pKa 8.5-9.5), His (pKa 6-7), Lys (pKa 10.5) and Ser (ROH, pKa 13).
An understanding of the chemical reactivity of the various R group side chains of the amino acids in a protein is important since chemical reagents that react specifically with a given amino acid side chain can be used to:
- identify the presence of the amino acids in unknown proteins or
- determine if a given amino acid is critical for the structure or function of the protein. For example, if a reagent that covalently interacts with only Lys is found to inhibit the function of the protein, a lysine might be considered to be important in the catalytic activity of the protein.
Figure: A REVIEW SUMMARY OF THE CHEMISTRY OF ALDEHYDES, KETONES, AND CARBOXYLIC ACID DERIVATIVES
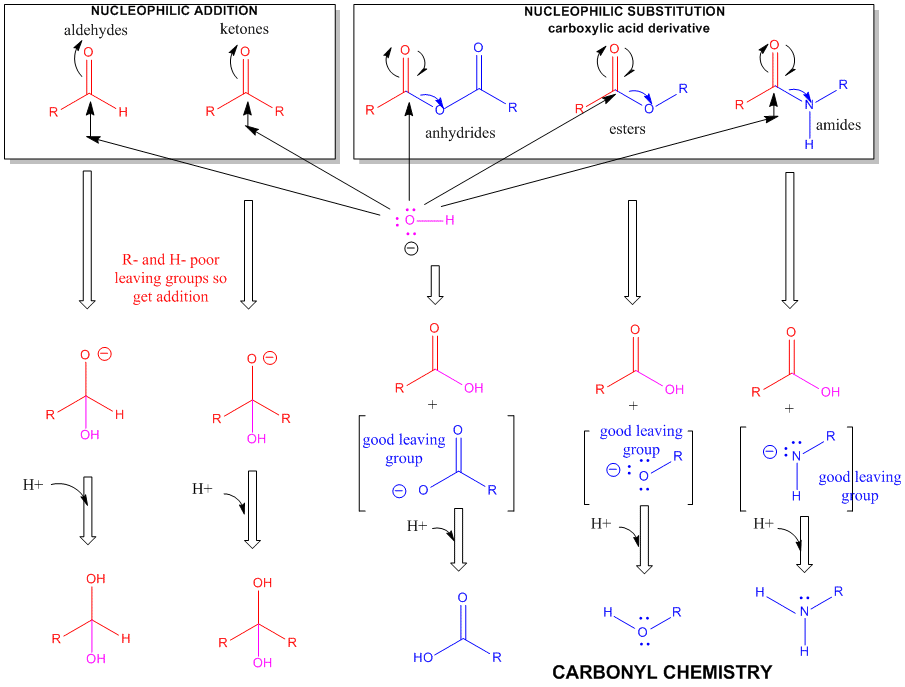
The side chain of serine is generally no more reactive than ethanol. It is a potent nucleophile in a certain class of proteins (proteases, for example) when it is deprotonated. The amino group of lysine is a potent nucleophile only when deprotonated.
Navigation
Return to Chapter 2A: Amino Acid Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 2A: Amino Acids

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.