Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 2 - PROTEIN STRUCTURE
A: AMINO ACIDS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 02/27/16
|
Learning Goals/Objectives for Chapter 2A: After class and this reading, students will be able to
|
A6. Reactions of Cysteine
Cysteine is a potent nucleophile, which is often linked to another Cys to form a covalent disulfide bond.
Figure: CYSTEINE REACTIONS 1
- reacts with iodoacetic acid in an SN2 rx., adding a carboxymethyl group to the S.
- reacts with iodoacetamide in an SN2 rx, adding a carboxyamidomethyl group to S.
- reacts with N-ethylmaleimide in an addition rx. to the double bond
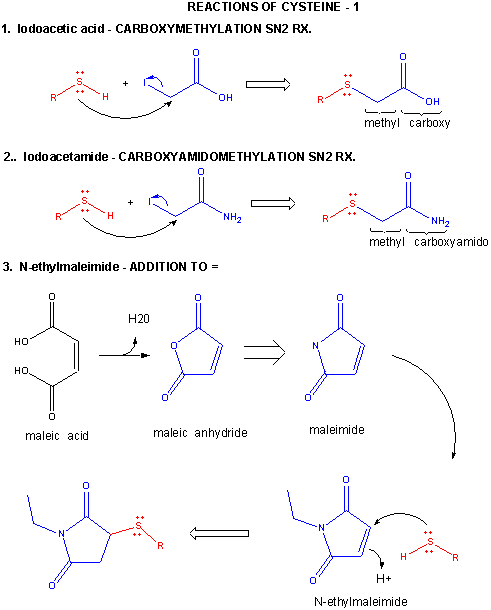
Figure: a quick review of
sulfur redox chemistry
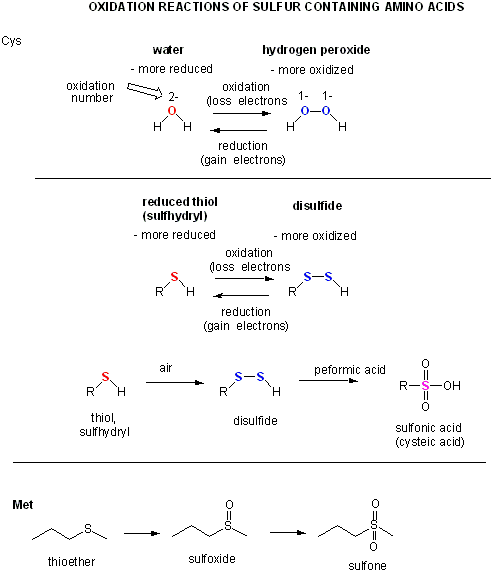
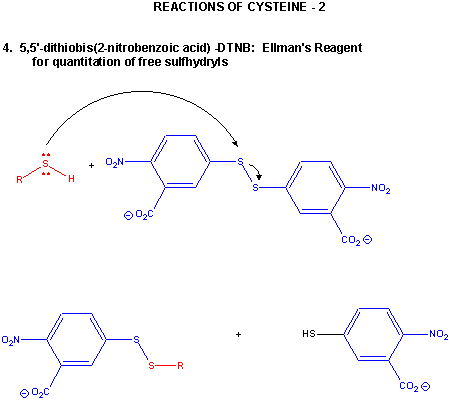
Figure: CYSTEINE REACTIONS 2
- reacts with R'-S-S-R'', a disulfide, in a disulfide interchange reaction, to form R-S-S-R'
- reacts with oxidizing agents like HCOOOH, performic acid, to form cysteic acid.
- reacts with 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB or Ellman's reagent) in a RSH displacement reaction in which DTNB is cleaved and the 2-nitro-5-thiobenzoic acid anion, which absorbs at 412 nm, is released. Used to quantitate total RSH in a protein
Navigation
Return to Chapter 2A: Amino Acid Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 2A: Amino Acids

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.