Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 2 - PROTEIN STRUCTURE
A: AMINO ACIDS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 02/27/16
|
Learning Goals/Objectives for Chapter 2A: After class and this reading, students will be able to
|
A7. Cystine Chemistry
Two cysteine side chains can covalently interact in a protein to produce a disulfide. Just as HOOH (hydrogen peroxide) is more oxidized than HOH (O in H2O2 has oxidation number of 1- while the O in H2O has an oxidation number of 2-) , RSSR is the oxidized form (S oxidation number 1-) and RSH is the reduced form (S oxidation number 2-) of thiols. There oxidation number are analogous since O and S are both in Group 6 of the periodic table and both are more electronegative than C.
A quick review of redox reactions and oxidation numbers.
Figure: DISULFIDE - CYSTINE - REACTIONS
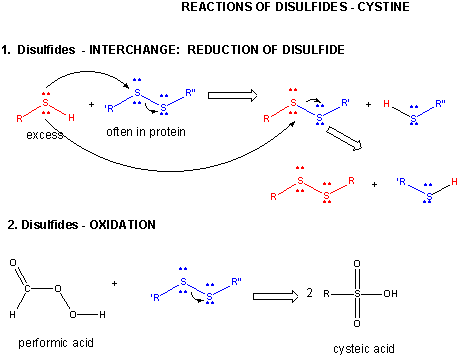
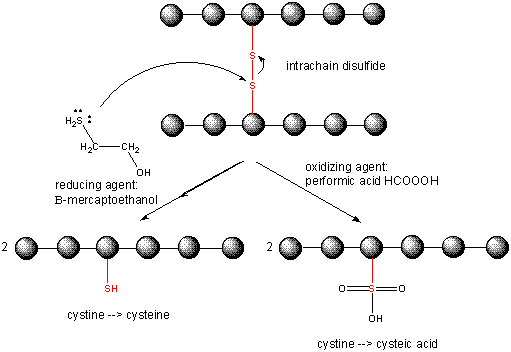
When a protein folds, two Cys side chains might approach each other, and form an intrachain disulfide bond. Likewise, two Cys side chains on separate proteins might approach each other and form an interchain disulfide. Such disulfides must be cleaved, and the chains separated before analyzing the sequence of the protein. The disulfide in protein can be cleaved by reducing agents such as beta-mercaptoethanol, dithiothreitol, tris (2-carboxyethyl) phosphine (TCEP) or oxidizing agents which further oxidizes the disulfide to separate cysteic acids.
Figure: Disulfide Oxidizing Agents - b-mercaptoethanol, dithiothreitol, and phosphines
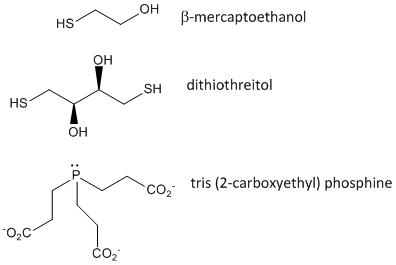
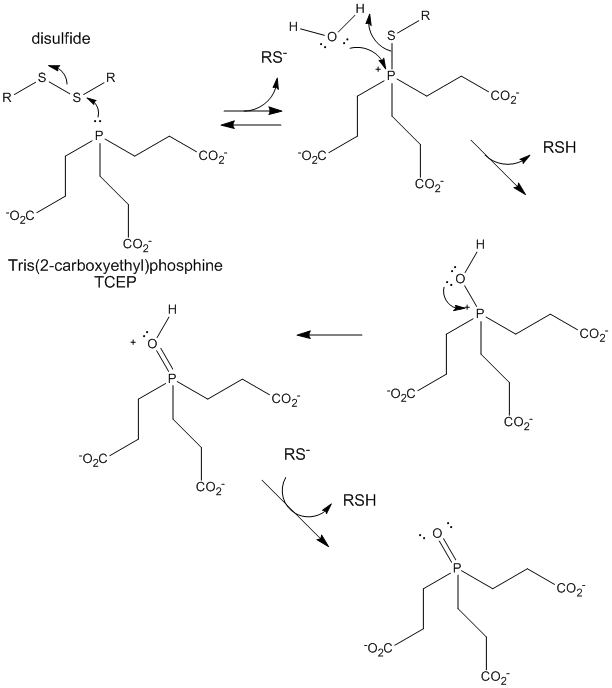
Figure: TCEP reduction of disulfides
The inside of cells are maintained in a reduced environment by the presence of many "reducing" agents, such as the tripeptide g-glu-cys-gly (glutathione). Hence intracellular proteins usually do not contain disulfides, which are abundant in extracellular proteins (such as those found in blood) or in certain organelles such as the endoplasmic reticulum and mitochondrial intermembrane space where disulfidesc can be introduced.
Figure:
Cleaving Disulfide Bonds in Proteins
Cysteine Redox Chemistry
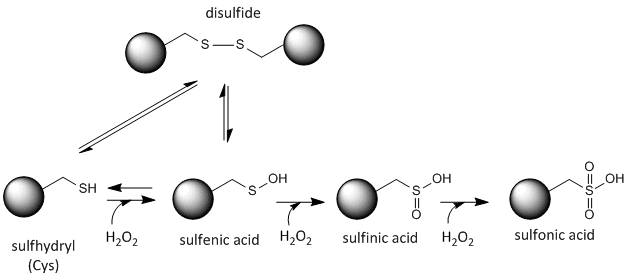
The sulfur in cysteine is redox-active and hence can exist in a wide variety of states, depending on the local redox environment and the presence of oxidzing and reducing agents. A potent oxidizing agent that can be made in cells is hydrogen peroxide, which can lead to more drastic and irreversible chemical modifications to the Cys side chains. If a reactive Cys is important to protein function, then the function of the protein can be modulated (sometimes reversibly, sometimes irreversibly) with various oxidizing agents, as shown in the figure below.
Figure: Redox state of Cysteine
Navigation
Return to Chapter 2A: Amino Acid Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 2A: Amino Acids

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.