Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 2 - PROTEIN STRUCTURE
A: AMINO ACIDS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 02/27/16
|
Learning Goals/Objectives for Chapter 2A: After class and this reading, students will be able to
|
A8. Reactions of Histidine
Histidine is one of the strongest bases at physiological pH's. The nitrogen atom in a secondary amine might be expected to be a stronger nucleophile than a primary amine through electron release to that N in a secondary amine. Opposing this effect is the steric hindrance by the two attached Cs of the N on attach on an electrophile . However, in His, this steric effect is minimized since the 2Cs are restrained by the ring. With a pKa of about 6.5, this amino acid is one of the strongest available bases at physiological pH (7.0). Hence, it can often cross-react with many of the reagents used to modify Lys side chains. His reacts with reasonably high selectivity with diethyl pyrocarbonate.
Figure: REACTIONS OF HISTIDINE
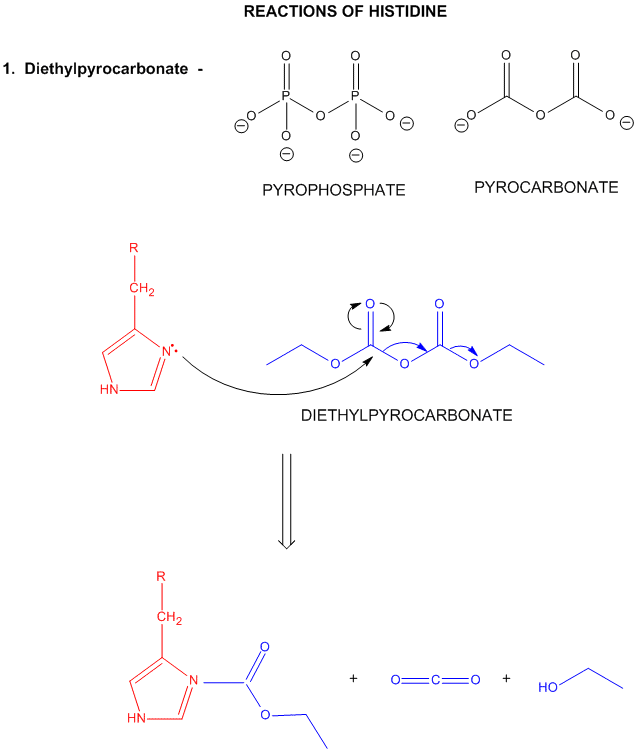
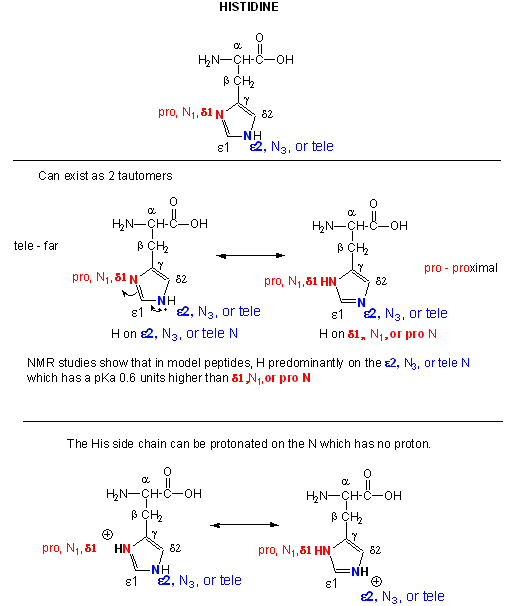
Figure: Where is the H on His? Where is the Charge?
Navigation
Return to Chapter 2A: Amino Acid Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 2A: Amino Acids

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.