Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - METABOLIC AND SIGNAL TRANSDUCTION
A. ACTIVE TRANSPORT
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9A:
|
A2. Transport of Ions
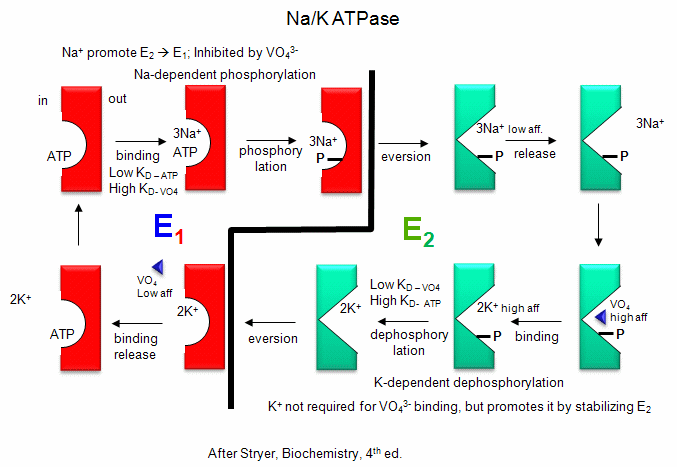
Na/K - These ions are both transported by the Na/K ATPase.
This protein keeps the K+in and Na+out high compared to their respective
concentrations on the other side of the membrane. The protein exists in two
essential conformations, E1 and E2, depending on the phosphorylation state
of the protein. ATP and 3 Na+ bind to the cytoplasmic domain of the enzyme
in the E1 conformation. In the presence of Na ions, the bound ATP is cleaved
in a nucleophilic atack by an Asp side chain of the protein. (Hence, the
protein is a Na+-activated ATPase. The phosphorylated enzyme changes
conformation to the E2 form in which Na+ ions are now on the outside of the
cell membrane, from which they dissociate. The phosphorylated protein in
conformation E2 now binds 2 K+ ions on the outside, which activates
hydrolysis of the Asp-PO3 mixed anhydride link. The dephosphorylated protein
is more stable in the E1 conformation to which it changes as it bring K+
ions into the cell. This is an example of an electrogenic antiporter.
Transport proteins that use this mechanism of transport are designated as P
types, since ATP cleavage is required and PO43- is covalenty transferred to
an Asp residue from the ATP. P-Type transporters are inhibited
by vanadate (VO43-), a transition state analog of phosphate. Note:
Transport mediated by P type membrane proteins can, in the lab, be used to
drive ATP synthesis.
Detailed kinetic analysis of ATP
and vanadate interactions show there are a low affinity and high affinity
site for each on Na/K ATPase. The high affinity vandate site appears
to be the same as the low affinity ATP site, which suggest that vandate
binds tightly to the E2 form of the enzyme. The low affinity vandate
site appears to be the same site (based on competition assays) as the ATP
site, which is probably the E1 form. Hence vandate binds
preferentially to the E2 form would inhibit the transition to the E1 form.
Vanadate also inhibits phosphatases, enymes that cleaves phosphorylated Ser,
Thr, and Tyr - phosphoesters in proteins. This supports the notion
that vanadate binds preferentially to the E2 form, which has a
phosphoanhydride link (Asp-O-phosphate) that is hydrolyzed, promoting the
conversion of E2 back to E1. Vanadate is probably at
transition state analog inhibitor in that it can readily adopt a trigonal
bipyramidal structure, mimicking the transition state for cleavage of the
tetrahedral anhydride bonds of ATP and Asp-O-PO4.
animation:
Na/K ATPase
Figure: Na/K-ATPase
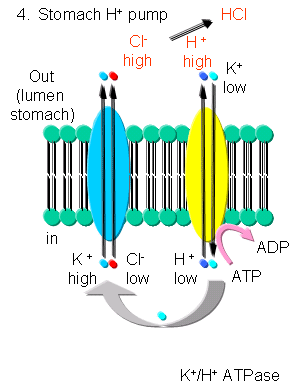
K - In addition to the above mechanism, K ions can be transported with protons in an electroneutral antiport mechanism by a K+/H+-ATPase found in stomach cells, which gives rise to a low pH in the lumen of the stomach.
Figure: K/H ATPase
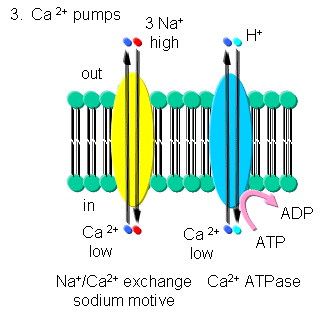
Ca - Calcium levels are very low in cells. Transient increases are more
likely to be detected in a signal transduction pathways than a transient
decrease in high basal or constituitive cytoplasmic levels. Ca2+-ATPase,
homologous to the Na/K-ATPase, removes Ca2+ from the cytoplasm to either the
outside of the cells or into internal organelles. In addition a Na+-Ca2+
exchange protein (an antiporter) transports calcium ions out of the cell
using a sodium-motive potential.
Transport of calcium ions
Figure: Transport of calcium ions
All of above ATPases are examples of P-type ion transporters.
Navigation
Return to Chapter 9A: Active Transport Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9A: Active Transport

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.